
Exhibit 99.1

COMPANY OVERVIEW July 2023
DISCLAIMERS Disclaimer . This presentation (“Presentation”) is preliminary in nature and for informational purposes only to assist interested parties in making their own evaluation with respect to the proposed business combination (the “Business Combination”) between BlueRiver Acquisition Corp . (“BlueRiver”) and Spinal Stabilization Technologies, LLC (“SST” or the “Company”) . The information contained herein does not purport to be all - inclusive and none of BlueRiver, the Company, Cohen & Company Capital Markets, nor any of their respective affiliates nor any of its or their control persons, officers, directors, employees or representatives makes any representation or warranty, express or implied, as to the accuracy, completeness or reliability of the information contained in this Presentation . You should consult your own counsel and tax and financial advisors as to legal and related matters concerning the matters described herein, and, by accepting this Presentation, you confirm that you are not relying upon the information contained herein to make any decision . You shall not rely upon any statement, representation or warranty made by any other person, firm or corporation (including, without limitation, Cohen & Company Capital Markets) or any of their respective affiliates or control persons, officers, directors and employees in making any investment decisions . None of BlueRiver, the Company, Cohen & Company Capital Markets, nor any of their respective affiliates nor any of its or their control persons, officers, directors, employees or representatives, shall be liable to any person for any information set forth herein or any action taken or not taken by any person, including any investment in shares of BlueRiver or the Company . Certain information contained in this Presentation relates to or is based on studies, publications, surveys and the Company’s own internal estimates and research . In addition, all of the market data included in this Presentation involves a number of assumptions and limitations, and there can be no guarantee as to the accuracy or reliability of such assumptions . Finally, while the Company believes its internal research is reliable, such research has not been verified by any independent source . This meeting and any information communicated at this meeting, including this Presentation, are strictly confidential and should not be discussed outside your organization . Forward - Looking Statements . Certain statements in this Presentation may be considered forward - looking statements . Forward - looking statements generally relate to future events or BlueRiver’s or the Company’s future financial or operating performance . For example, statements concerning the following include forward - looking statements : the Company’s plans to execute on its business plan, begin FDA trial enrollment, and commercialize PerQdisc ; the efficacy of PerQdisc ; the Company’s U . S . and international market opportunity ; the design, timing and results of the Company’s clinical trials ; the viability of the Company’s growth and commercialization strategy, including related capabilities ; trends and developments in the medical device industry ; the advantages and potential of the Company’s solution ; its visibility into future financial performance ; and the total addressable markets for the Company’s medical device . In some cases, you can identify forward - looking statements by terminology such as “may”, “should”, “expect”, “intend”, “will”, “estimate”, “anticipate”, “believe”, “predict”, “potential” or “continue”, or the negatives of these terms or variations of them or similar terminology . Forward - looking statements are subject to risks, uncertainties, and other factors which could cause actual results to differ materially from those expressed or implied by such forward looking statements . These forward - looking statements are based upon estimates and assumptions that, while considered reasonable by BlueRiver and its management, and the Company and its management, as the case may be, are inherently uncertain . New risks and uncertainties may emerge from time to time, and it is not possible to predict all risks and uncertainties . Such factors include, but are not limited to, the Company’s dependence upon successful completion of regulatory approvals in a time when the push for comparative research models may increase the time and cost to get a device to market . Additional risks include the Company’s limited operating history and history of significant operating losses ; fluctuations in quarterly operating results, which are difficult to predict ; the Company’s dependence on developing new products or product enhancements ; challenges associated with complying with applicable state, federal and international regulations related to sales of medical devices and governing the Company’s relationships with physicians and other consultants ; prior studies' results may not be predicative of later studies' outcomes ; inability to obtain, retain or reacquire certifications, including our CE Mark that was suspended ; and the Company’s ability to compete with large, well - established medical device manufacturers with significant resources . You should not place undue reliance on forward - looking statements in this Presentation, which speak only as of the date they are made and are qualified in their entirety by reference to the cautionary statements herein . Neither BlueRiver nor the Company undertakes any duty to update these forward - looking statements . You should carefully consider the risks and uncertainties described in the “Risk Factors” section of BlueRiver’s most recent Annual Report on Form 10 - K, any proxy statement/prospectus relating to the Business Combination, which is expected to be filed by BlueRiver with the SEC, other documents filed by BlueRiver from time to time with the SEC and any risk factors made available to you in connection with BlueRiver, the Company and the Business Combination . CONFIDENTIAL | APM008 rev. A; DCO0029 2
DISCLAIMERS (CONT’D) No Offer or Solicitation . This Presentation and the information contained herein do not constitute (i) (a) a solicitation of a proxy, consent or authorization with respect to any securities or in respect of the proposed Business Combination or (b) an offer to sell or the solicitation of an offer to buy any security, commodity or instrument or related derivative, nor shall there be any sale of securities in any jurisdiction in which the offer, solicitation or sale would be unlawful prior to the registration or qualification under the securities laws of any such jurisdiction, or (ii) an offer or commitment to lend, syndicate or arrange a financing, underwrite or purchase or act as an agent or advisor or in any other capacity with respect to any transaction, or commit capital, or to participate in any trading strategies . No offering of securities shall be made except by means of a prospectus meeting the requirements of Section 10 of the Securities Act of 1933 , as amended, or an exemption therefrom . The distribution of this Presentation may also be restricted by law and persons into whose possession this Presentation comes should inform themselves about and observe any such restrictions . The recipient acknowledges that it is (a) aware that the United States securities laws prohibit any person who has material, non - public information concerning a company from purchasing or selling securities of such company or from communicating such information to any other person under circumstances in which it is reasonably foreseeable that such person is likely to purchase or sell such securities, and (b) familiar with the Securities Exchange Act of 1934 , as amended, and the rules and regulations promulgated thereunder (collectively, the “Exchange Act”), and that the recipient will neither use, nor cause any third party to use, this Presentation or any information contained herein in contravention of the Exchange Act, including, without limitation, Rule 10 b - 5 thereunder . NEITHER THE SECURITIES AND EXCHANGE COMMISSION (“SEC”) NOR ANY STATE OR TERRITORIAL SECURITIES COMMISSION HAS APPROVED OR DISAPPROVED OF THE SECURITIES OR DETERMINED IF THIS PRESENTATION IS TRUTHFUL OR COMPLETE . Trademarks . BlueRiver and the Company own or have rights to various trademarks, service marks and trade names that they use in connection with the operation of their respective businesses . This Presentation may also contain trademarks, service marks, trade names and copyrights of other companies, which are the property of their respective owners . The use or display of third parties’ trademarks, service marks, trade names or products in this Presentation is not intended to, and does not imply, a relationship with BlueRiver or the Company, or an endorsement or sponsorship by or of BlueRiver or the Company . Solely for convenience, some of the trademarks, service marks, trade names and copyrights referred to in this Presentation may be listed without the TM, SM, © or ® symbols, but such references are not intended to indicate, in any way, that BlueRiver or the Company will not assert, to the fullest extent under applicable law, their rights or the rights of the applicable owners, if any, to these trademarks, service marks, trade names and copyrights . Additional Information . In connection with the proposed Business Combination, BlueRiver intends to file with the SEC a registration statement on Form F - 4 containing a preliminary proxy statement/prospectus of BlueRiver, and after the registration statement is declared effective, BlueRiver will mail a definitive proxy statement/prospectus relating to the proposed Business Combination to its shareholders . This Presentation does not contain all the information that should be considered concerning the proposed Business Combination and is not intended to form the basis of any investment decision or any other decision in respect of the Business Combination . BlueRiver’s shareholders and other interested persons are advised to read, when available, the preliminary proxy statement/prospectus and the amendments thereto and the definitive proxy statement/prospectus and other documents filed in connection with the proposed Business Combination, as these materials will contain important information about the Company, BlueRiver and the Business Combination . When available, the definitive proxy statement/prospectus and other relevant materials for the proposed Business Combination will be mailed to shareholders of BlueRiver as of a record date to be established for voting on the proposed Business Combination . Shareholders will also be able to obtain copies of the preliminary proxy statement/prospectus, the definitive proxy statement/prospectus and other documents filed with the SEC, without charge, once available, at the SEC’s website at www . sec . gov, or by directing a request to : BlueRiver Acquisition Corp . , 250 West Nottingham Drive, Suite 400 , San Antonio, TX 78209 . Participants in the Solicitation . BlueRiver, the Company and their respective directors and executive officers may be deemed participants in the solicitation of proxies from BlueRiver’s shareholders with respect to the proposed Business Combination . A list of the names of BlueRiver’s directors and executive officers and a description of their interests in BlueRiver is contained in BlueRiver’s most recent Annual Report on Form 10 - K, and in BlueRiver’s definitive proxy statement filed on January 13 , 2023 , which were filed with the SEC and are available free of charge at the SEC’s web site at www . sec . gov, or by directing a request to BlueRiver Acquisition Corp . , 250 West Nottingham Drive, Suite 400 , San Antonio, TX 78209 . Additional information regarding the interests of the participants in the solicitation of proxies from BlueRiver’s shareholders with respect to the proposed Business Combination will be contained in the proxy statement/prospectus for the proposed Business Combination when available . Investors and security holders of BlueRiver and the Company are urged to read the proxy statement/prospectus and other relevant documents that will be filed with the SEC carefully and in their entirety when they become available because they will contain important information about the proposed Business Combination . Investors and security holders will be able to obtain free copies of the proxy statement/prospectus and other documents containing important information about BlueRiver and the Company through the website maintained by the SEC at www . sec . gov . Copies of the documents filed with the SEC by BlueRiver can be obtained free of charge by directing a written request to BlueRiver Acquisition Corp . , 250 West Nottingham Drive, Suite 400 , San Antonio, TX 78209 . CONFIDENTIAL | APM008 rev. A; DCO0029 3

BLUERIVER OVERVIEW: INVESTORS WITH DEEP OPERATING AND PUBLIC MARKETS EXPE RIENCE CONFIDENTIAL | APM008 rev. A; DCO0029 • Experienced team with combination of deep investing, operating and transactional experience • Extensive and deep network of owner, board, corporate executive, family office and CEO - level relationships • Track record of shareholder value creation via both organic growth and capitalizing on transactional opportunities at the right times • Long - term growth objectives with focus on adding value post - acquisition over the investment lifecycle 4 OVERVIEW OF BLUERIVER INDEPENDENT BOARD AND TEAM Anne Farlow – Board Member • Non - Executive Chairman of the Board of Pershing Square Holdings, Ltd • Prior roles at Electra Partners, Morgan Stanley and Bain and Company John E. Sununu – Board Member • Served as a U.S. Senator from New Hampshire from 2003 to 2009 • Director of Boston Scientific since 2009, Council member of Lloyd’s of London since 2019, former director of Time Warner Cable Alok Sama – Board Member • Former President, Softbank Group International • Senior Advisor, Warburg Pincus, Raine Group • Director, ARM, Fortress Group, Airtel Africa Eric Medina – Managing Director • Experienced private equity investment professional with prior roles at Westhook Capital, aPriori Capital Partners and DLJ Merchant Banking Partners Randall Mays – Co - Chairman, Co - CEO and CFO • Founder & CEO of Running M Capital and Co - Managing Partner of Mays Family Enterprises • Various leadership roles including Vice Chairman, President and CFO of Clear Channel Communications • Board of Live Nation Entertainment, Digital Defense, BuildGroup Technology Fund, Live Undiscovered Music (LÜM), Mind Science Foundation and the Mays Family Foundation • Previously served on the boards of CNET, XM Satellite Radio, and American Tower John Gregg – Co - Chairman and Co - CEO • Founder of Bluewater Ventures • Completed over 50 acquisitions, divestitures and corporate restructurings and arranged over $18 billion in strategic equity investments • Served on the board of over 25 companies. Previously CFO for NTL Inc (now Virgin Media), prior leadership roles at Cellular Communications, Iesy GMBH and Cablecom GMBH

Transaction • Spinal Stabilization Technologies LLC (“SST” or the “Company”) to combine with BlueRiver Acquisition Corp . (“BlueRiver”) • Transaction implies a pre money equity value of $ 240 . 0 million and a pro forma enterprise value of $ 301 . 9 million • Existing SST shareholders to retain 100 % of their equity and will own ~ 70 % of the pro forma company at closing • Target closing Q 4 2023 Proceeds • BlueRiver expects to raise approximately $ 40 million for SST prior to the closing of the business combination • Use of proceeds includes cash to balance sheet, which will be used to execute on the Company’s business plan, begin the FDA pivotal trial enrollment and commercial expansion TRANSACTION SUMMARY CONFIDENTIAL | APM008 rev. A; DCO0029 5
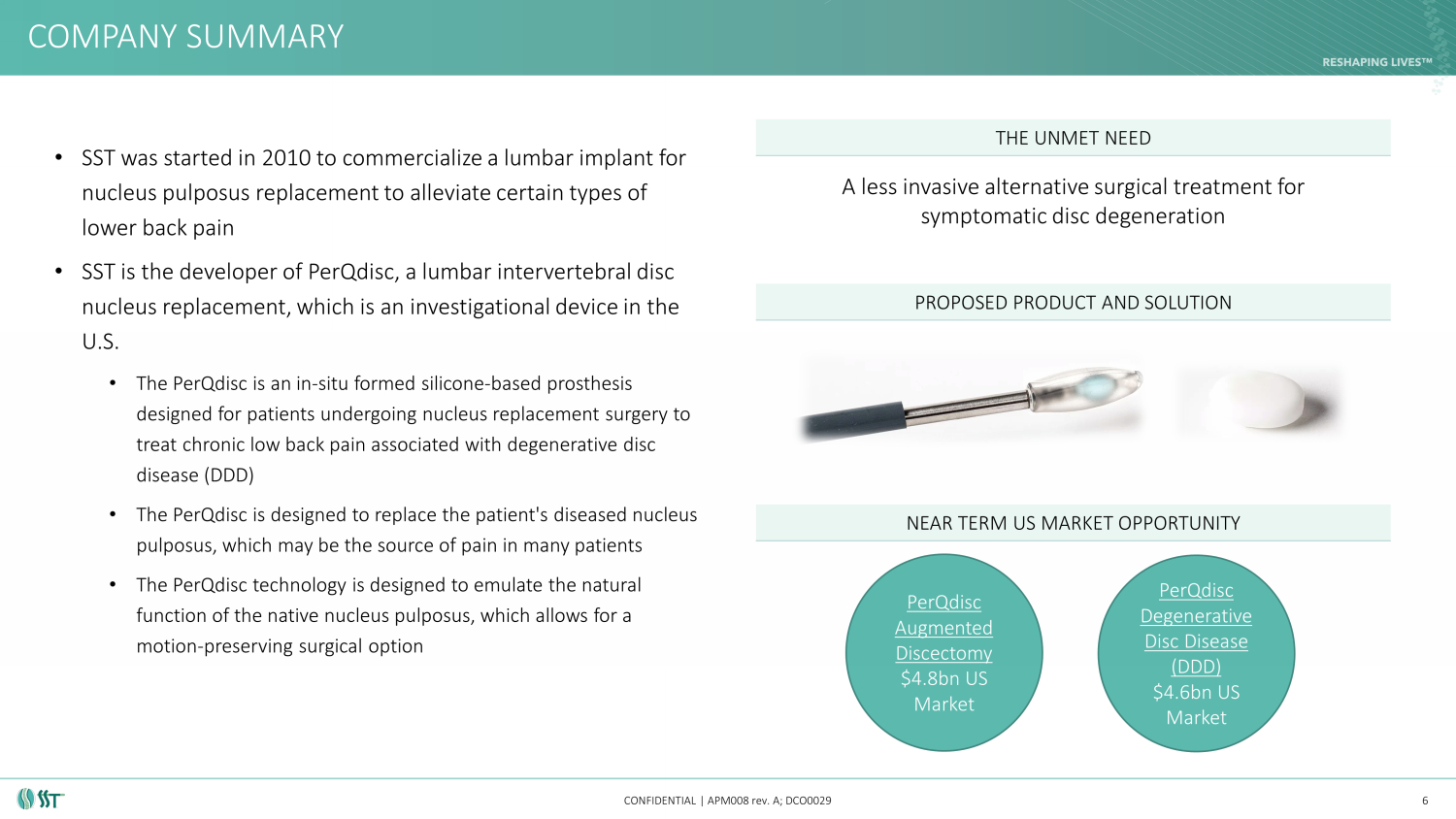
• SST was started in 2010 to commercialize a lumbar implant for nucleus pulposus replacement to alleviate certain types of lower back pain • SST is the developer of PerQdisc , a lumbar intervertebral disc nucleus replacement, which is an investigational device in the U.S. • The PerQdisc is an in - situ formed silicone - based prosthesis designed for patients undergoing nucleus replacement surgery to treat chronic low back pain associated with degenerative disc disease (DDD) • The PerQdisc is designed to replace the patient's diseased nucleus pulposus, which may be the source of pain in many patients • The PerQdisc technology is designed to emulate the natural function of the native nucleus pulposus, which allows for a motion - preserving surgical option A less invasive alternative surgical treatment for symptomatic disc degeneration COMPANY SUMMARY CONFIDENTIAL | APM008 rev. A; DCO0029 THE UNMET NEED PROPOSED PRODUCT AND SOLUTION NEAR TERM US MARKET OPPORTUNITY 6 PerQdisc Augmented Discectomy $4.8bn US Market PerQdisc Degenerative Disc Disease (DDD) $4.6bn US Market
PRESIDENT AND CHIEF EXECUTIVE OFFICER VICE PRESIDENT R&D AND OPERATIONS CFO, GENERAL MANAGER AND CONTROLLER, SST IRELAND DIRECTOR OF REGULATORY AND QUALITY MANAGEMENT MEDICAL DIRECTOR • Over 30 years medical device experience • Over 20 years experience developing spine technology • Over 30 years global financial management experience • Over 10 years experience in the medical device sector • Over 15 years experience in neurological surgery SST MANAGEMENT TEAM CONFIDENTIAL | APM008 rev. A; DCO0029 MARK NOVOTNY LOREN FRANCIS BRIAN DOWLING MOLLY BOND DR. JEFF GOLAN 7

MARK NOVOTNY CASE STUDY CONFIDENTIAL | APM008 rev. A; DCO0029 8 Critical Care • Former program manager at Ohmeda • Developed a family of patient monitors used in general anesthesia and critical care Interventional Cardiology • Former Vice President at Boston Scientific • Program manger for the TAXUS stent in charge of the US launch strategy • Launched TAXUS in all international markets. • Integrated the Watchman program into the new BSC structural heart program • Innovation leader in wires, balloons, hearth valves and IVUS imagining technologies Mitral Valve Repair System • Developing a catheter - based mitral valve repair technology as Co - Founder of CardioMech Spinal Stabilization Technologies • Developing a lumbar implant to alleviate certain types of lower back pain as President and CEO of SST Over 30 years of bench to bedside innovation around billion - dollar medical technology products

SST ADVISORY BOARD CONFIDENTIAL | APM008 rev. A; DCO0029 Background • Director Spine Surgery, Department of Orthopaedic Surgery, Rush University Medical Center • Professor of Orthopaedic Surgery, Rush University Medical Center • Co - Director Spine Fellowship, Rush University Medical Center • Head, Section of Minimally Invasive Spine Surgery, Rush University Medical Center • Founder, board member and past president of the Society of Minimally Invasive Spine Surgery • Served as the director of the Spine Center at The University of Chicago and former Spine Fellowship Director and associate professor at The University of Chicago Background • Professor of Surgery, Dept . of Surgery, Cedars - Sinai Medical Center, Los Angeles, CA • Medical Director, Director of Spine Education Spine Center, Div . Orthopaedic Surgery, Dept . of Surgery, Cedars - Sinai Medical Center, Los Angeles, CA • Medical Director, Director of Research Center for Spinal Restoration (CSR) & The Spine Institute Santa Monica, CA • Former Co - Fellowship Director Spine Center, Div . Orthopaedic Surgery, Dept . of Surgery, Cedars - Sinai Medical Center, Los Angeles, CA • Former Spine Surgeon, Director of Research The Spine Institute at Saint John’s Health Center, Santa Monica, CA Background • Internationally renowned expert in the field of minimal - invasive spine surgery and interventional pain therapy • Founded InsightSpine and is offering a comprehensive spinal disc pain service at The London Clinic and KIMS Hospital Maidstone • Co - surgeon of the worldwide first successful full - endoscopic nucleus replacement surgery in 2009 and principal investigator of international multicentre studies for a novel nucleus implant • Past President of the German Interventional Spine Society (GIW e . V . ), and serves as Master Instructor for Spine Intervention Society (SIS, USA) 9 DR. FRANK PHILLIPS ORTHOPAEDIC SURGEON DR. HYUN W. BAE ORTHOPAEDIC SURGEON DR. MICHAEL HESS ORTHOPAEDIC SURGEON DR. LUIS TUMIALÁN ORTHOPAEDIC SURGEON Background • Professor of Neurosurgery at the Barrow Neurological Institute specializing in minimally invasive spinal surgery • Director of minimally invasive spine surgery, Barrow Brain and Spine • Performed over 1 , 200 minimally invasive motion preserving lumbar and cervical decompressions and more than 800 minimally invasive lumbar fusions • Author of the definitive textbook on Minimally Invasive Spinal Surgery used by residents, fellows and surgeons around the country and the world and author of more than 70 published peer reviewed journal articles DR. CIARAN BOLGER ORTHOPAEDIC SURGEON Background • Council of the Society of British Neurosurgeons • President and Chairman of the European Spine Foundation • UK representative to both the European Association of Neurosurgical Societies and the World Federation on Neurosurgical Societies • Invited Member of the Spine Society of Australia and a member of the North American Spine Society • Previously served in the British Cervical Spine Society • Past Secretary and Past President of EuroSpine (the Spine Society of Europe) and the current Meeting President of the European Association of Neurosurgical Societies
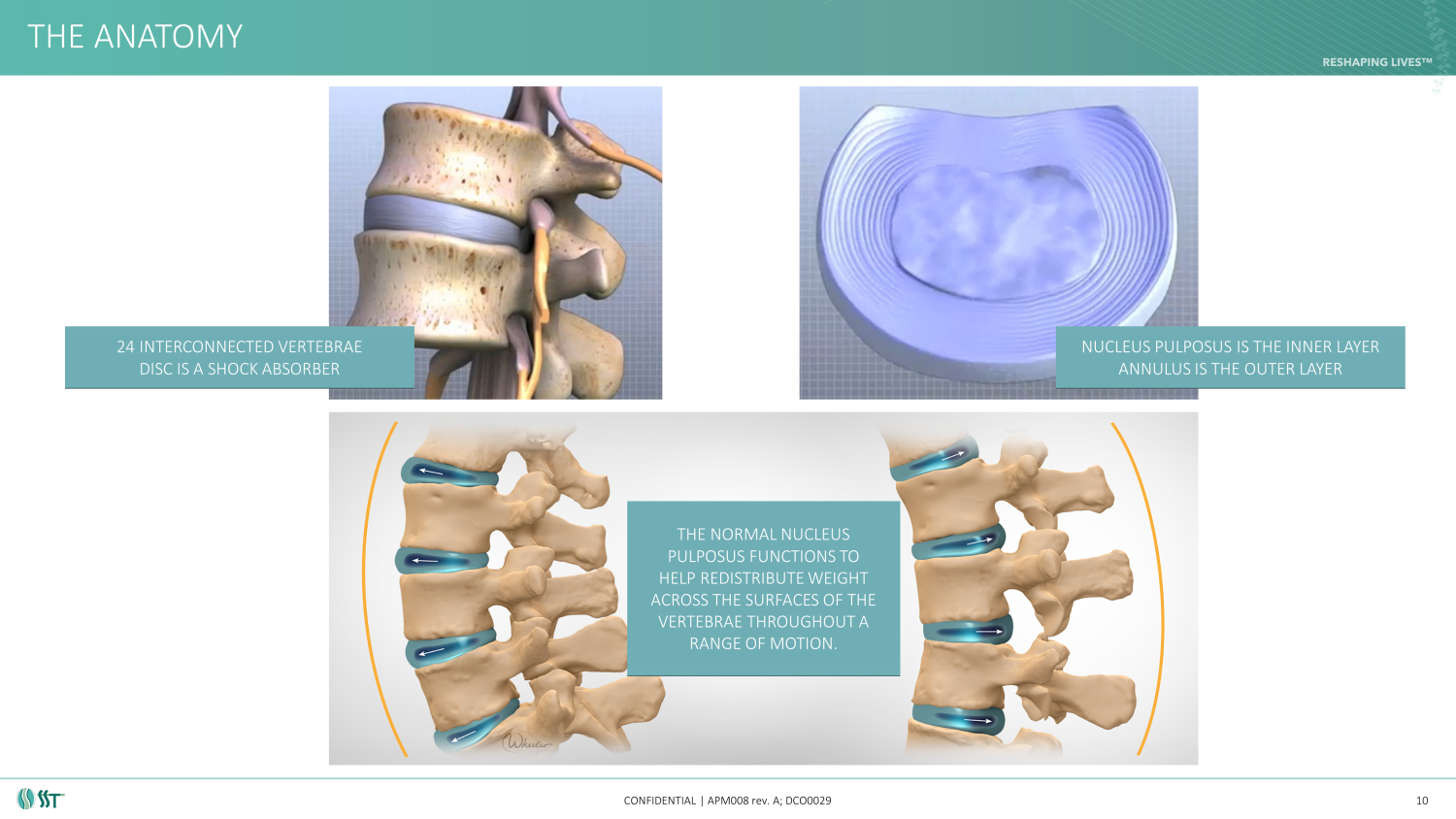
THE ANATOMY CONFIDENTIAL | APM008 rev. A; DCO0029 10 24 INTERCONNECTED VERTEBRAE DISC IS A SHOCK ABSORBER NUCLEUS PULPOSUS IS THE INNER LAYER ANNULUS IS THE OUTER LAYER THE NORMAL NUCLEUS PULPOSUS FUNCTIONS TO HELP REDISTRIBUTE WEIGHT ACROSS THE SURFACES OF THE VERTEBRAE THROUGHOUT A RANGE OF MOTION.
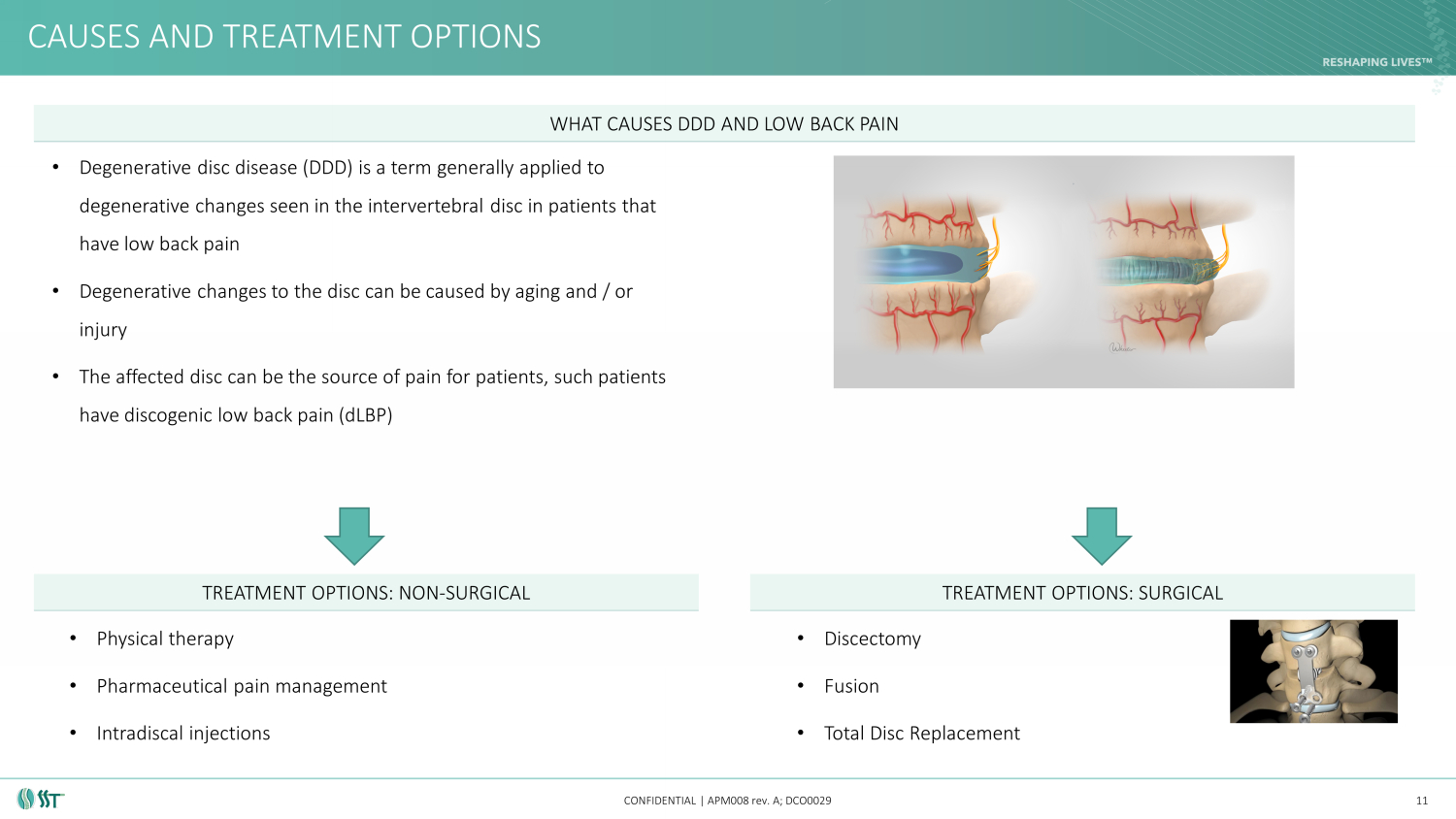
CAUSES AND TREATMENT OPTIONS CONFIDENTIAL | APM008 rev. A; DCO0029 11 • Physical therapy • Pharmaceutical pain management • Intradiscal injections • Discectomy • Fusion • Total Disc Replacement TREATMENT OPTIONS: NON - SURGICAL TREATMENT OPTIONS: SURGICAL WHAT CAUSES DDD AND LOW BACK PAIN • Degenerative disc disease (DDD) is a term generally applied to degenerative changes seen in the intervertebral disc in patients that have low back pain • Degenerative changes to the disc can be caused by aging and / or injury • The affected disc can be the source of pain for patients, such patients have discogenic low back pain ( dLBP )
INDICATION FOR USE: DEGENERATIVE DISC DISEASE TWO PROPOSED INDICATIONS/ONE DEVICE (1) CONFIDENTIAL | APM008 rev. A; DCO0029 The SST PerQdisc Nucleus Replacement System is intended to replace the nucleus pulposus of the intervertebral disc in the L1 - S1 spinal region in patients with single level discogenic pain. The patient may have single or multi - level degenerative disc disease (DDD) but the discogenic pain must be limited to a single level. The patient must be skeletally mature, at least 21 years of age, presenting with moderate to severe low back pain (with or without leg pain) that is unresponsive to non - surgical conservative treatments. The patient should not have a history of prior lumbar spine surgery. The patient should have a minimum disc height of 6 mm. 12 INDICATION FOR USE: EXPANDED INDICATIONS (AUGMENTED DISCECTOMY) The SST PerQdisc Nucleus Replacement System is intended to replace the nucleus pulposus and support the annulus fibrosis of the intervertebral disc in the L1 - S1 spinal region in patients with symptomatic radiculopathy from a focal lumbar disc herniation that undergo partial discectomy and/or sequestrectomy. The patient must be skeletally mature, at least 21 years of age, presenting with symptomatic radiculopathy from focal disc herniation. The patient should not have a history of prior lumbar spine surgery at the index level. The patient should have a minimum central disc height of 6 mm. (1) Degenerative Disc Disease is a proposed Indication for use and Augmented Discectomy is a proposed expanded indication.
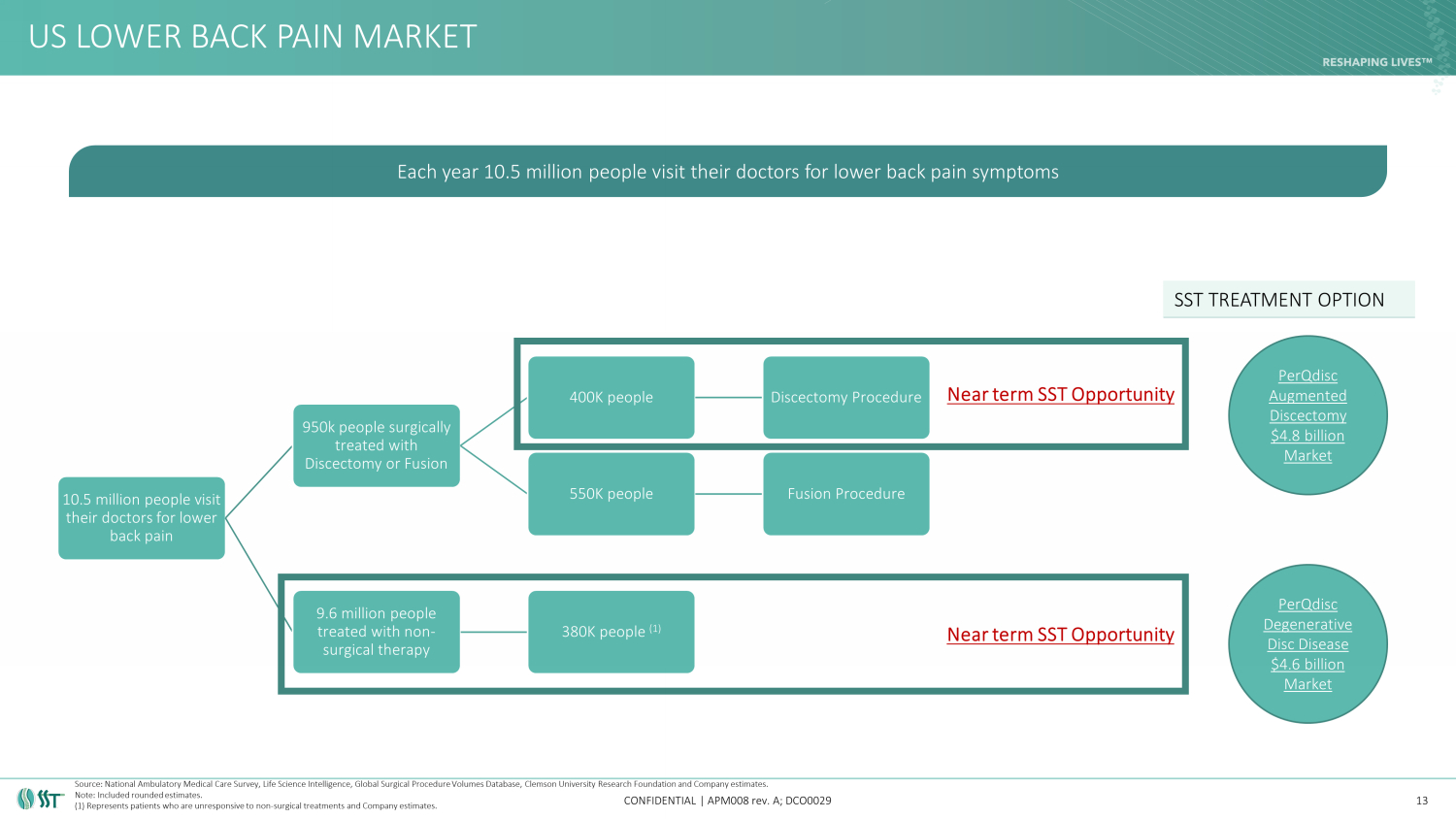
US LOWER BACK PAIN MARKET CONFIDENTIAL | APM008 rev. A; DCO0029 13 10.5 million people visit their doctors for lower back pain 950k people surgically treated with Discectomy or Fusion 400K people Discectomy Procedure 550K people Fusion Procedure 9.6 million people treated with non - surgical therapy 380K people (1) Near term SST Opportunity Each year 10.5 million people visit their doctors for lower back pain symptoms Source: National Ambulatory Medical Care Survey, Life Science Intelligence, Global Surgical Procedure Volumes Database, Clems on University Research Foundation and Company estimates. Note: Included rounded estimates. (1) Represents patients who are unresponsive to non - surgical treatments and Company estimates. Near term SST Opportunity PerQdisc Augmented Discectomy $4.8 billion Market SST TREATMENT OPTION PerQdisc Degenerative Disc Disease $4.6 billion Market
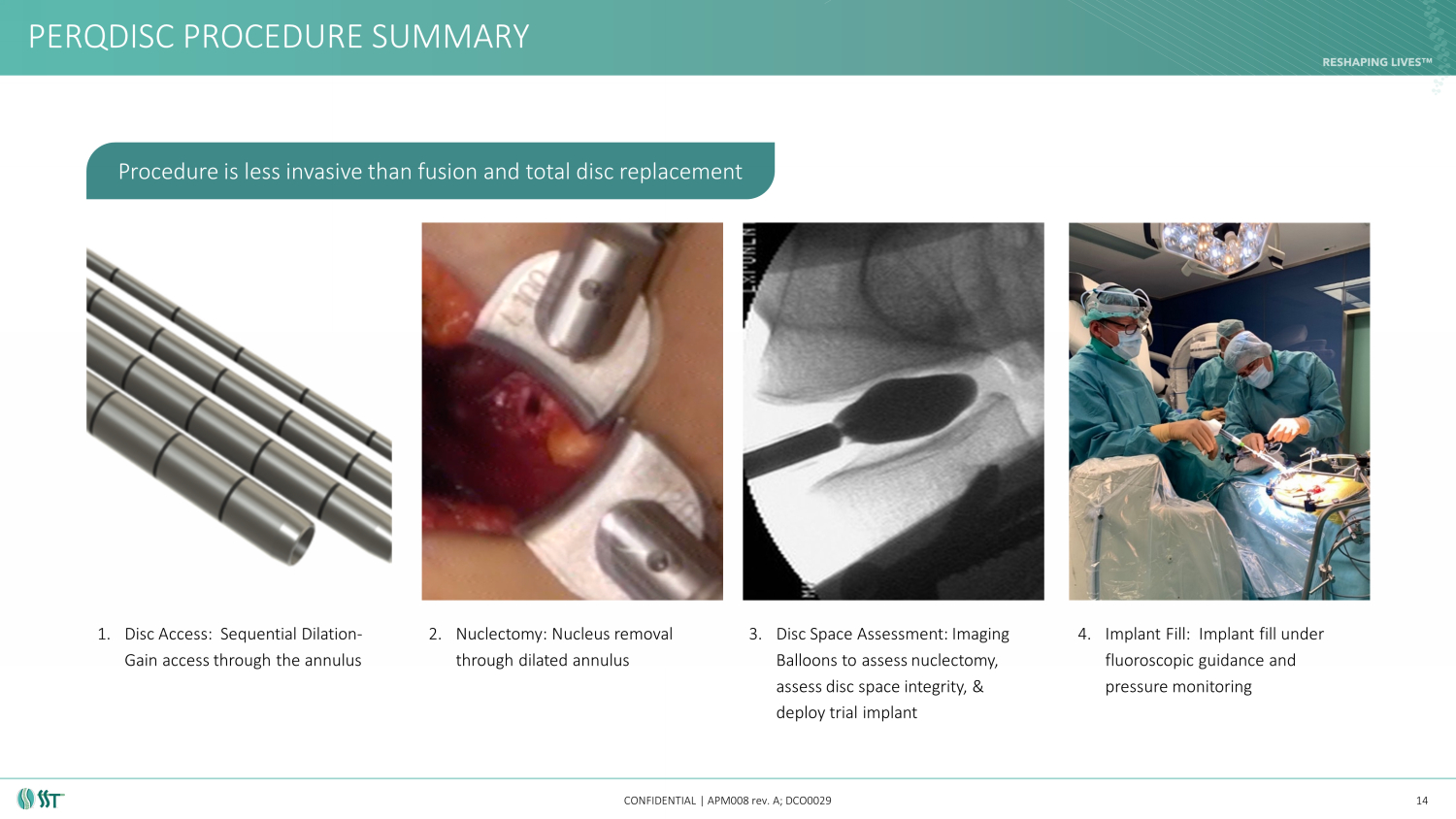
PERQDISC PROCEDURE SUMMARY CONFIDENTIAL | APM008 rev. A; DCO0029 14 Procedure is less invasive than fusion and total disc replacement 1. Disc Access: Sequential Dilation - Gain access through the annulus 2. Nuclectomy : Nucleus removal through dilated annulus 3. Disc Space Assessment: Imaging Balloons to assess nuclectomy , assess disc space integrity, & deploy trial implant 4. Implant Fill: Implant fill under fluoroscopic guidance and pressure monitoring
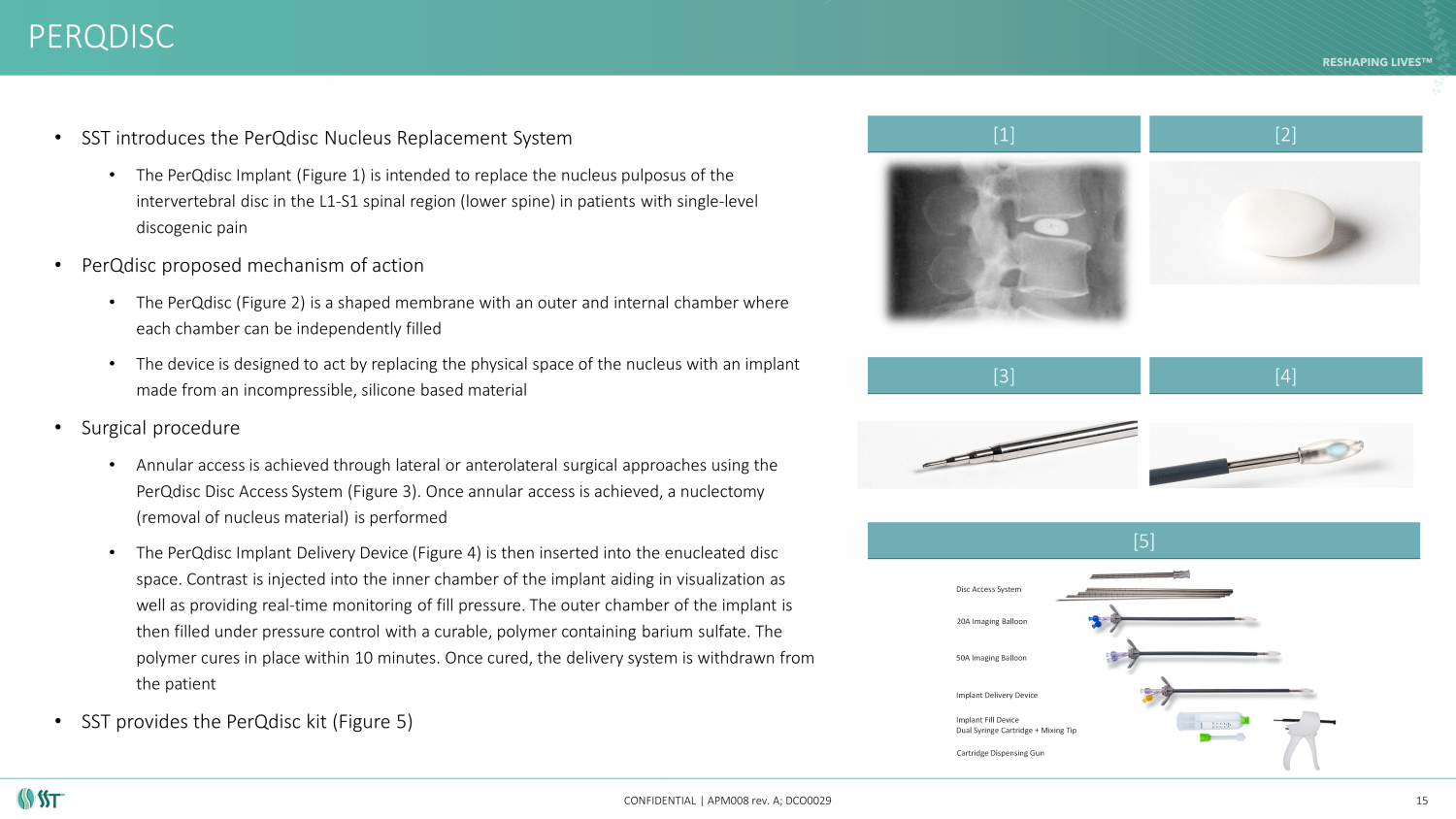
PERQDISC CONFIDENTIAL | APM008 rev. A; DCO0029 15 • SST introduces the PerQdisc Nucleus Replacement System • The PerQdisc Implant (Figure 1) is intended to replace the nucleus pulposus of the intervertebral disc in the L1 - S1 spinal region (lower spine) in patients with single - level discogenic pain • PerQdisc proposed mechanism of action • The PerQdisc (Figure 2) is a shaped membrane with an outer and internal chamber where each chamber can be independently filled • The device is designed to act by replacing the physical space of the nucleus with an implant made from an incompressible, silicone based material • Surgical procedure • Annular access is achieved through lateral or anterolateral surgical approaches using the PerQdisc Disc Access System (Figure 3). Once annular access is achieved, a nuclectomy (removal of nucleus material) is performed • The PerQdisc Implant Delivery Device (Figure 4) is then inserted into the enucleated disc space. Contrast is injected into the inner chamber of the implant aiding in visualization as well as providing real - time monitoring of fill pressure. The outer chamber of the implant is then filled under pressure control with a curable, polymer containing barium sulfate. The polymer cures in place within 10 minutes. Once cured, the delivery system is withdrawn from the patient • SST provides the PerQdisc kit (Figure 5) [1] [2] [3] [4] [5]
SST PATENT PORTFOLIO CONFIDENTIAL | APM008 rev. A; DCO0029 16 Summary • 8 patent families in the portfolio with 65 active patents/applications • Coverage extends to current implant and other potential products • Note that the 65 patents includes nationalized European patents • 14 issued and 1 allowed US patents ; 3 issued European patents ; 4 issued Chinese patents ; 3 issued Japanese patents ; 4 issued Australian patents • Priority dates from 2006 - 2019 ; projected expiry from 2026 - 2039 • International Filings • SST has selectively filed corresponding patent applications in commercially relevant jurisdictions, including, for example, in Australia, Canada, China, Europe, South Korea and the U . S .
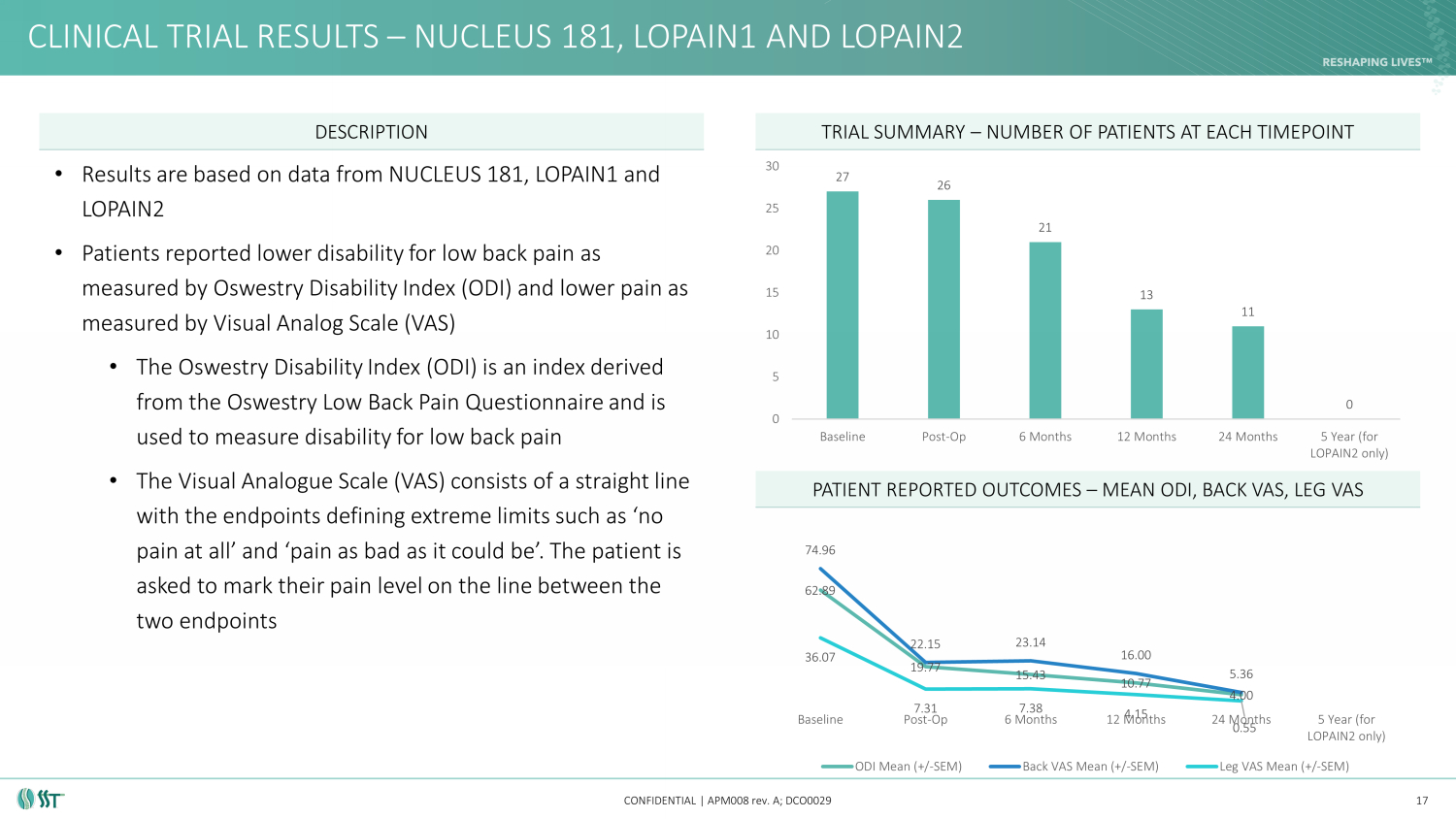
CLINICAL TRIAL RESULTS – NUCLEUS 181, LOPAIN1 AND LOPAIN2 CONFIDENTIAL | APM008 rev. A; DCO0029 17 DESCRIPTION TRIAL SUMMARY – NUMBER OF PATIENTS AT EACH TIMEPOINT PATIENT REPORTED OUTCOMES – MEAN ODI, BACK VAS, LEG VAS 62.89 19.77 15.43 10.77 4.00 74.96 22.15 23.14 16.00 5.36 36.07 7.31 7.38 4.15 0.55 Baseline Post-Op 6 Months 12 Months 24 Months 5 Year (for LOPAIN2 only) ODI Mean (+/-SEM) Back VAS Mean (+/-SEM) Leg VAS Mean (+/-SEM) 27 26 21 13 11 0 0 5 10 15 20 25 30 Baseline Post-Op 6 Months 12 Months 24 Months 5 Year (for LOPAIN2 only) • Results are based on data from NUCLEUS 181, LOPAIN1 and LOPAIN2 • Patients reported lower disability for low back pain as measured by Oswestry Disability Index (ODI) and lower pain as measured by Visual Analog Scale (VAS) • The Oswestry Disability Index (ODI) is an index derived from the Oswestry Low Back Pain Questionnaire and is used to measure disability for low back pain • The Visual Analogue Scale (VAS) consists of a straight line with the endpoints defining extreme limits such as ‘no pain at all’ and ‘pain as bad as it could be’. The patient is asked to mark their pain level on the line between the two endpoints
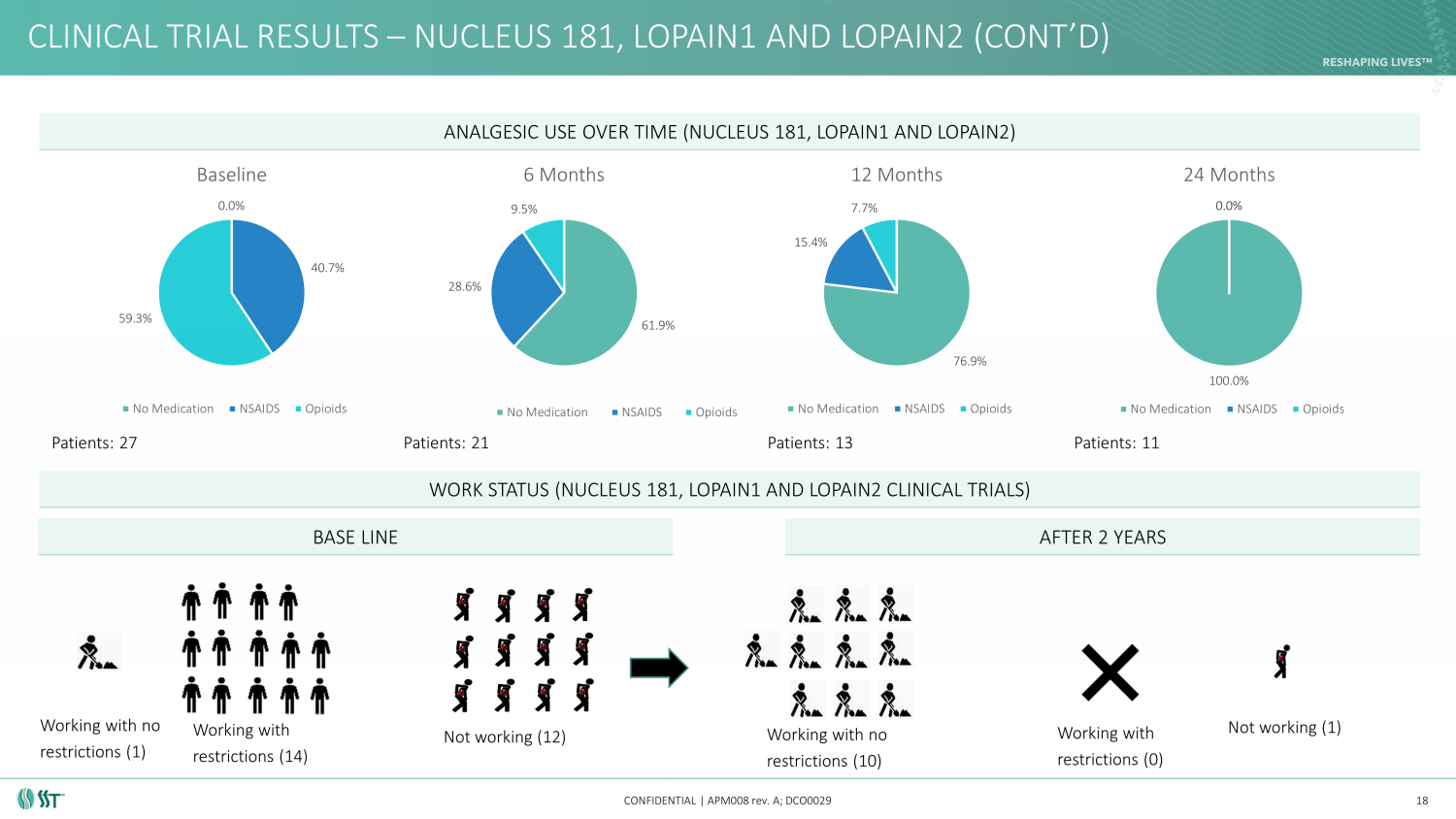
CLINICAL TRIAL RESULTS – NUCLEUS 181, LOPAIN1 AND LOPAIN2 (CONT’ D) CONFIDENTIAL | APM008 rev. A; DCO0029 18 ANALGESIC USE OVER TIME (NUCLEUS 181, LOPAIN1 AND LOPAIN2) WORK STATUS (NUCLEUS 181, LOPAIN1 AND LOPAIN2 CLINICAL TRIALS) 61.9% 28.6% 9.5% 6 Months No Medication NSAIDS Opioids 76.9% 15.4% 7.7% 12 Months No Medication NSAIDS Opioids 100.0% 0.0% 0.0% 24 Months No Medication NSAIDS Opioids 0.0% 40.7% 59.3% Baseline No Medication NSAIDS Opioids Working with no restrictions (1) Working with restrictions (14) Not working (12) Working with no restrictions (10) Not working (1) BASE LINE AFTER 2 YEARS Patients: 27 Patients: 21 Patients: 13 Patients: 11 Working with restrictions (0)

CERTIFICATIONS AND REGULATORY MILESTONES CONFIDENTIAL | APM008 rev. A; DCO0029 19 Q170356/S003 e Trade/Device Name: PerQdisc Nucleus Replacement System Received: March 4, 2021 We are pleased to inform you that your device and proposed indication for use meet the criteria and have been granted designation as a Breakthrough Device. Please refer to the FDA guidance document entitled " Breakthrough Devices Program ", for more information regarding the program, available at https:// www.fda.gov /media/108135/download Sincerely, Ronald P. Jean, Ph.D . Director DHT6B: Division of Spinal Devices OHT6: Office of Orthopedic Devices Office of Product Evaluation and Quality Center for Devices and Radiological Health Received ISO 13485 Certification – April 2 , 2021 CE Mark Granted May 20, 2021 CER being updated to remove CE suspension limitation Breakthrough Device Designation Granted Working with the FDA on IDE Design
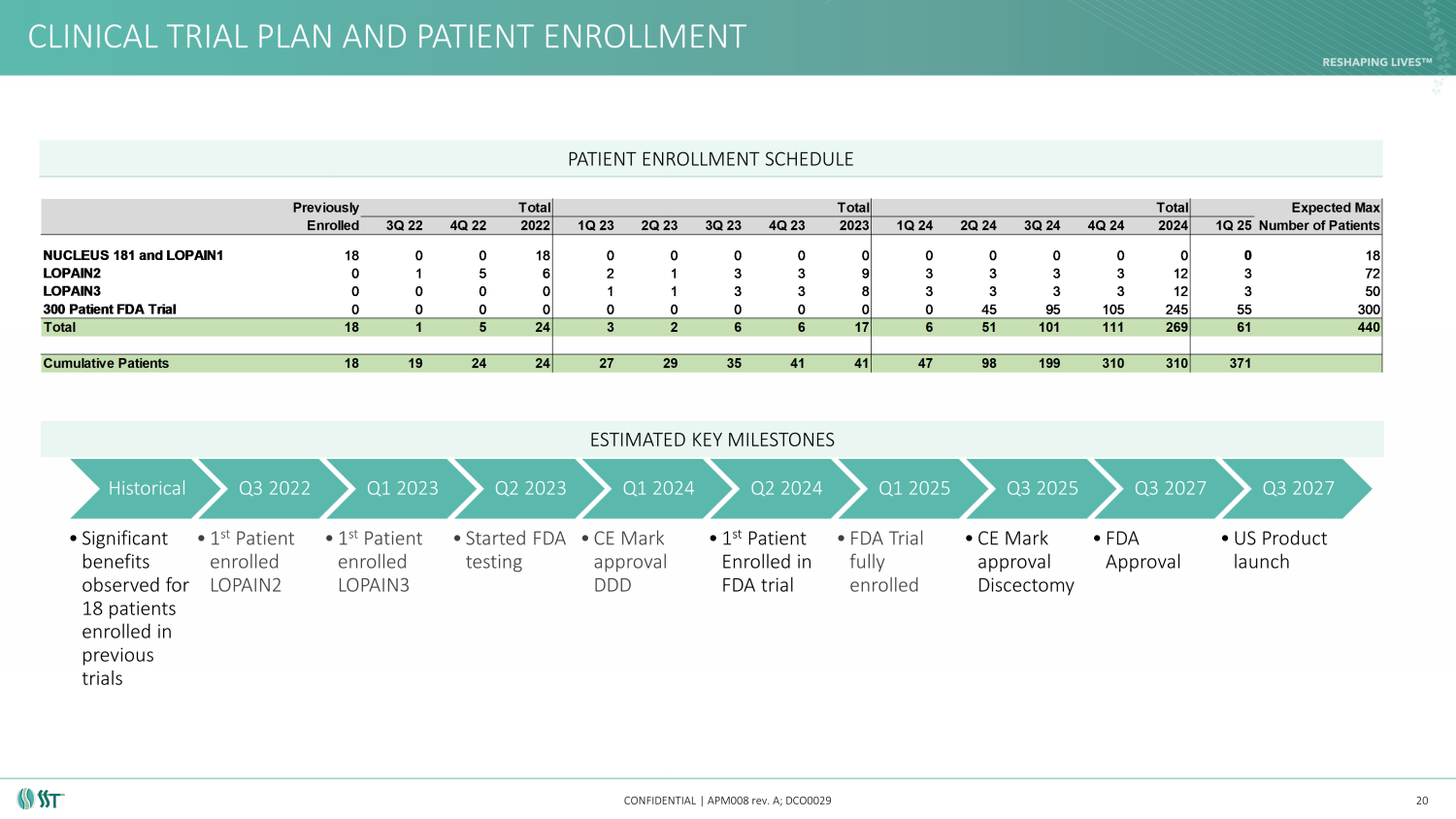
CLINICAL TRIAL PLAN AND PATIENT ENROLLMENT CONFIDENTIAL | APM008 rev. A; DCO0029 20 ESTIMATED KEY MILESTONES Historical • Significant benefits observed for 18 patients enrolled in previous trials Q3 2022 • 1 st Patient enrolled LOPAIN2 Q1 2023 • 1 st Patient enrolled LOPAIN3 Q2 2023 • Started FDA testing Q1 2024 • CE Mark approval DDD Q2 2024 • 1 st Patient Enrolled in FDA trial Q1 2025 • FDA Trial fully enrolled Q3 2025 • CE Mark approval Discectomy Q3 2027 • FDA Approval Q3 2027 • US Product launch PATIENT ENROLLMENT SCHEDULE Previously Total Total Total Expected Max Enrolled 3Q 22 4Q 22 2022 1Q 23 2Q 23 3Q 23 4Q 23 2023 1Q 24 2Q 24 3Q 24 4Q 24 2024 1Q 25Number of Patients NUCLEUS 181 and LOPAIN1 18 0 0 18 0 0 0 0 0 0 0 0 0 0 0 18 LOPAIN2 0 1 5 6 2 1 3 3 9 3 3 3 3 12 3 72 LOPAIN3 0 0 0 0 1 1 3 3 8 3 3 3 3 12 3 50 300 Patient FDA Trial 0 0 0 0 0 0 0 0 0 0 45 95 105 245 55 300 Total 18 1 5 24 3 2 6 6 17 6 51 101 111 269 61 440 Cumulative Patients 18 19 24 24 27 29 35 41 41 47 98 199 310 310 371
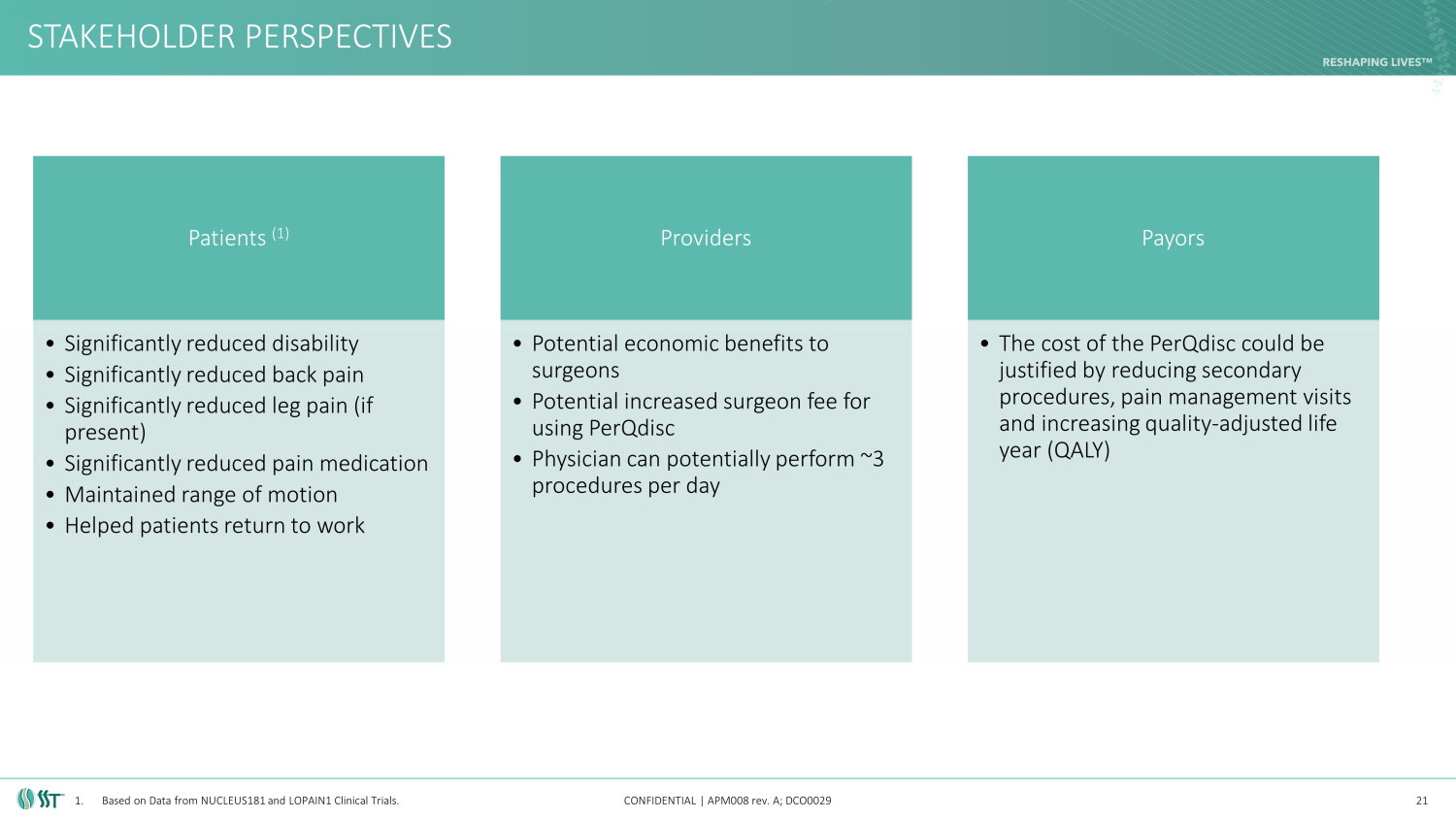
STAKEHOLDER PERSPECTIVES CONFIDENTIAL | APM008 rev. A; DCO0029 21 Patients (1) • Significantly reduced disability • Significantly reduced back pain • Significantly reduced leg pain (if present) • Significantly reduced pain medication • Maintained range of motion • Helped patients return to work Providers • Potential economic benefits to surgeons • Potential increased surgeon fee for using PerQdisc • Physician can potentially perform ~3 procedures per day Payors • The cost of the PerQdisc could be justified by reducing secondary procedures, pain management visits and increasing quality - adjusted life year (QALY) 1. Based on Data from NUCLEUS181 and LOPAIN1 Clinical Trials.
Large Market Opportunity Patent Portfolio Clinical Trials In Progress Business Model Differentiation M&A Opportunities Strong Leadership and Sponsorship INVESTMENT HIGHLIGHTS CONFIDENTIAL | APM008 rev. A; DCO0029 22

Regulatory Affairs and Clinical Trial Program
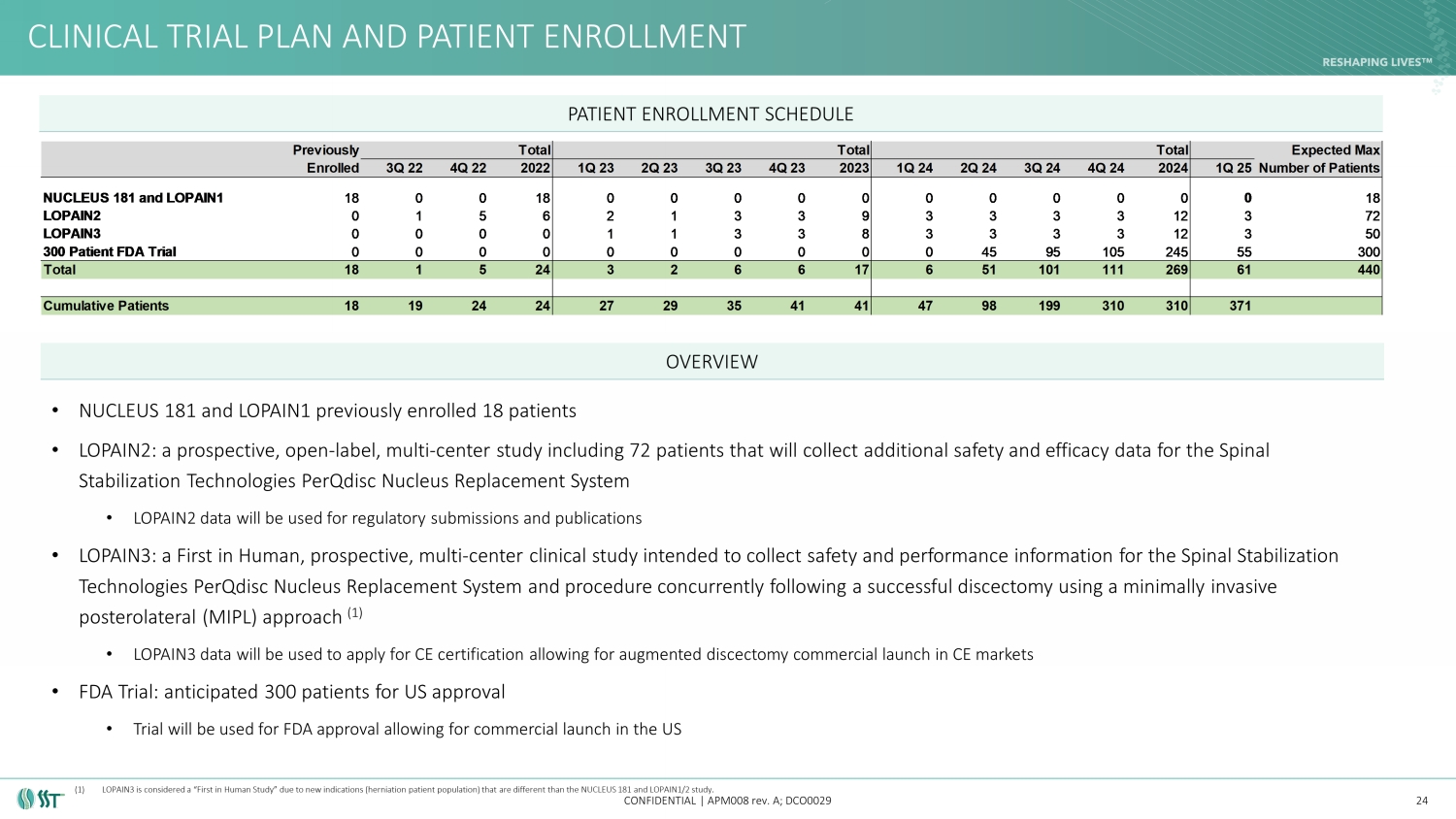
CLINICAL TRIAL PLAN AND PATIENT ENROLLMENT CONFIDENTIAL | APM008 rev. A; DCO0029 • NUCLEUS 181 and LOPAIN1 previously enrolled 18 patients • LOPAIN2: a prospective, open - label, multi - center study including 72 patients that will collect additional safety and efficacy da ta for the Spinal Stabilization Technologies PerQdisc Nucleus Replacement System • LOPAIN2 data will be used for regulatory submissions and publications • LOPAIN3: a First in Human, prospective, multi - center clinical study intended to collect safety and performance information for t he Spinal Stabilization Technologies PerQdisc Nucleus Replacement System and procedure concurrently following a successful discectomy using a minimally invasive posterolateral (MIPL) approach (1) • LOPAIN3 data will be used to apply for CE certification allowing for augmented discectomy commercial launch in CE markets • FDA Trial: 300 patients for US approval • Trial will be used for FDA approval allowing for commercial launch in the US 24 PATIENT ENROLLMENT SCHEDULE OVERVIEW Previously Total Total Total Expected Max Enrolled 3Q 22 4Q 22 2022 1Q 23 2Q 23 3Q 23 4Q 23 2023 1Q 24 2Q 24 3Q 24 4Q 24 2024 1Q 25Number of Patients NUCLEUS 181 and LOPAIN1 18 0 0 18 0 0 0 0 0 0 0 0 0 0 0 18 LOPAIN2 0 1 5 6 2 1 3 3 9 3 3 3 3 12 3 72 LOPAIN3 0 0 0 0 1 1 3 3 8 3 3 3 3 12 3 50 300 Patient FDA Trial 0 0 0 0 0 0 0 0 0 0 45 95 105 245 55 300 Total 18 1 5 24 3 2 6 6 17 6 51 101 111 269 61 440 Cumulative Patients 18 19 24 24 27 29 35 41 41 47 98 199 310 310 371 (1) LOPAIN3 is considered a “First in Human Study” due to new indications (herniation patient population) that are different than th e NUCLEUS 181 and LOPAIN1/2 study.

PERQDISC RESULTS (1) CONFIDENTIAL | APM008 rev. A; DCO0029 25 • Significantly reduced disability ( Oswestry Disability Index or “ODI”) • Average reduction in ODI from 59.3 to 4.0 for patients that reached the 2 year follow up • Significantly reduced back pain • Average reduction in Back VAS from 72.7 to 5.4 for patients that reached the 2 year follow up • Significantly reduced leg pain (if present) • Average reduction in Leg VAS from 37.9 to 0.5 for patients that reached the 2 year follow up • Significantly reduced level of pain medication • For patients that reached the 2 year follow up, no patients were taking opioids • Maintained range of motion (“ RoM ”) • There was no significant difference in RoM between screening and 24 months • Helped patients return to work • For patients that reached the 2 year follow up, 91% were working without restrictions 1. Based on Data from NUCLEUS181 & LOPAIN1 Clinical Trials.
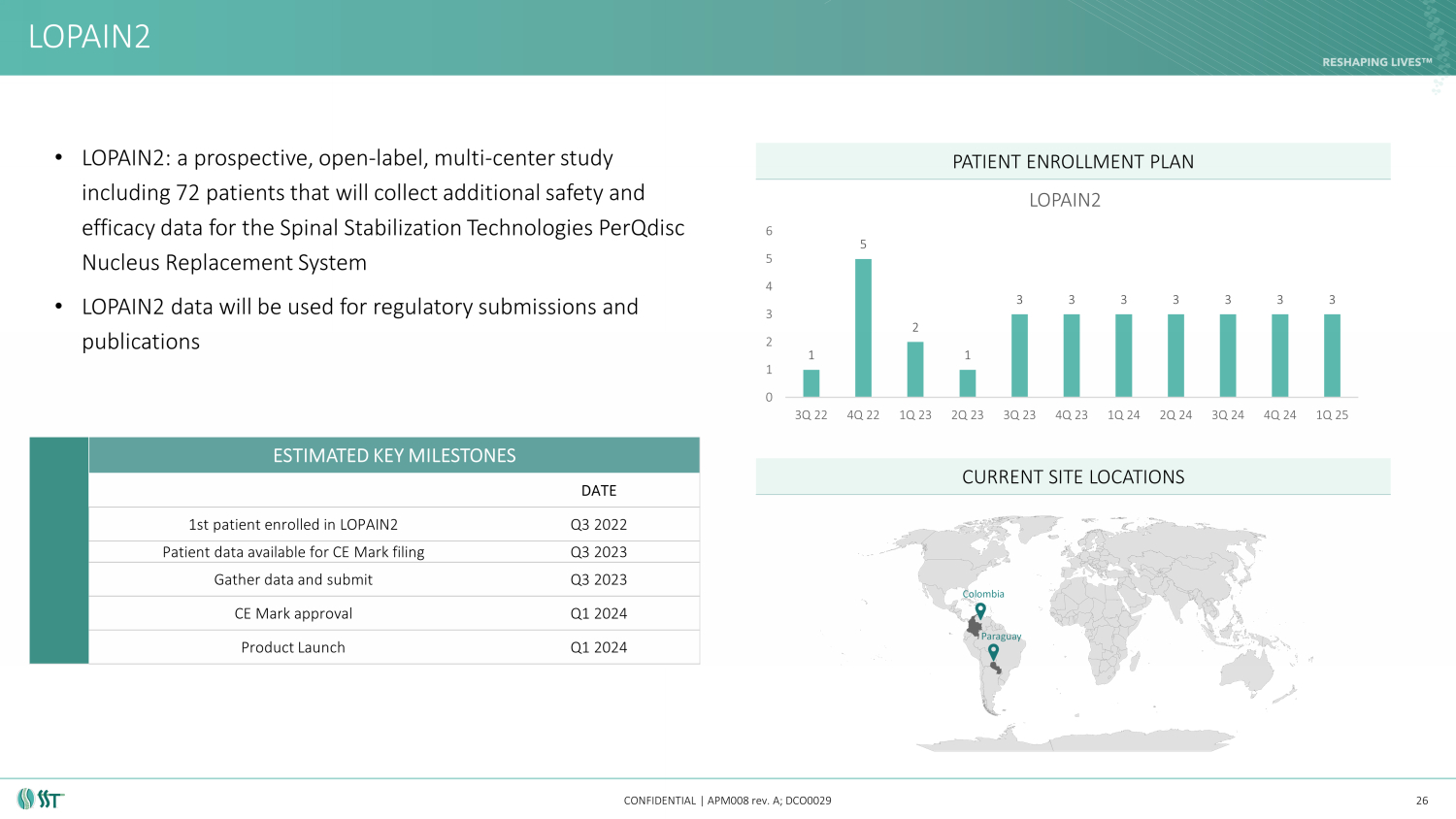
• LOPAIN2: a prospective, open - label, multi - center study including 72 patients that will collect additional safety and efficacy data for the Spinal Stabilization Technologies PerQdisc Nucleus Replacement System • LOPAIN2 data will be used for regulatory submissions and publications LOPAIN2 CONFIDENTIAL | APM008 rev. A; DCO0029 PATIENT ENROLLMENT PLAN CURRENT SITE LOCATIONS 26 ESTIMATED KEY MILESTONES DATE 1st patient enrolled in LOPAIN2 Q3 2022 Patient data available for CE Mark filing Q3 2023 Gather data and submit Q3 2023 CE Mark approval Q1 2024 Product Launch Q1 2024 Paraguay Colombia 1 5 2 1 3 3 3 3 3 3 3 0 1 2 3 4 5 6 3Q 22 4Q 22 1Q 23 2Q 23 3Q 23 4Q 23 1Q 24 2Q 24 3Q 24 4Q 24 1Q 25 LOPAIN2
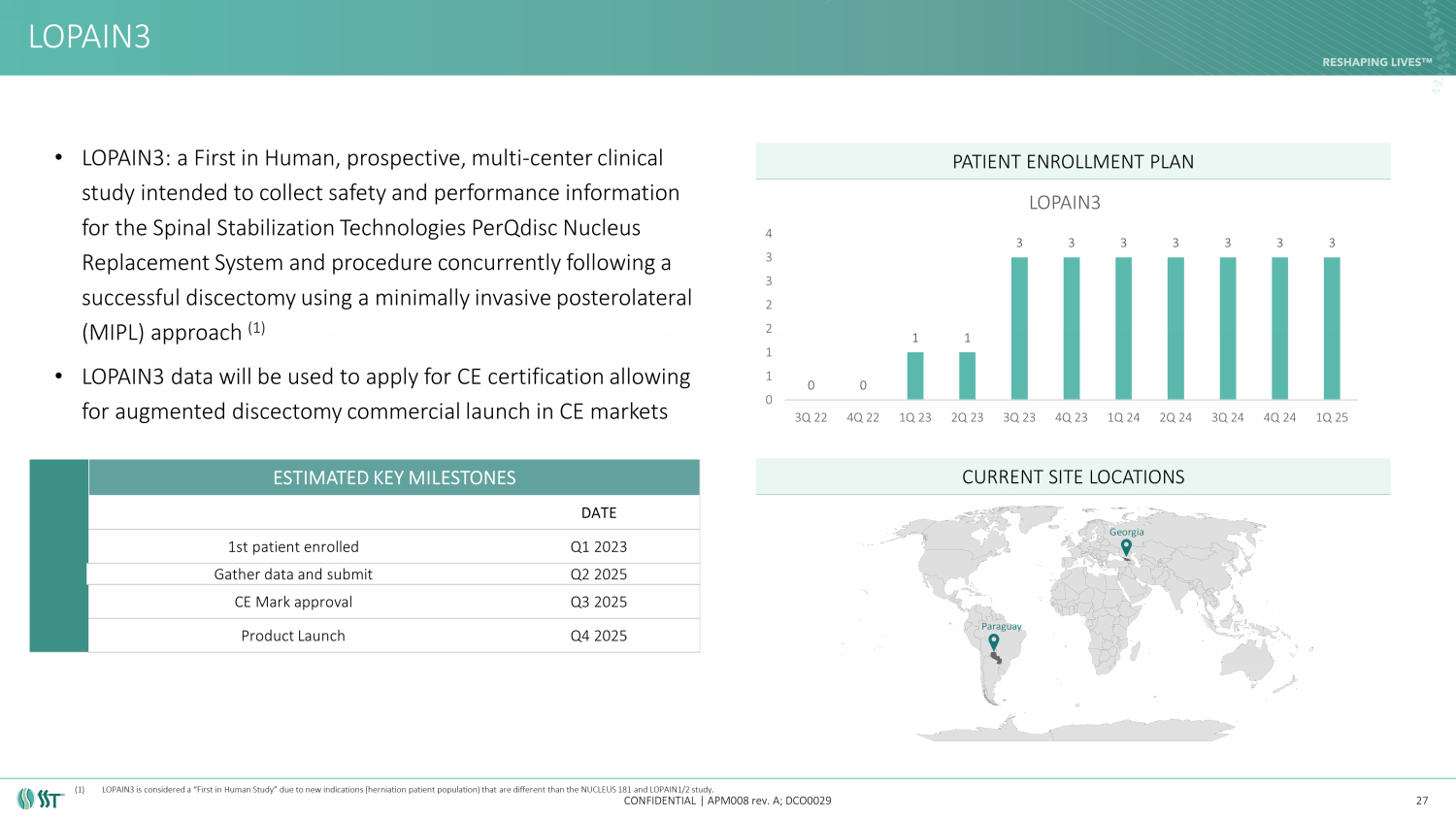
• LOPAIN3: a First in Human, prospective, multi - center clinical study intended to collect safety and performance information for the Spinal Stabilization Technologies PerQdisc Nucleus Replacement System and procedure concurrently following a successful discectomy using a minimally invasive posterolateral (MIPL) approach (1) • LOPAIN3 data will be used to apply for CE certification allowing for augmented discectomy commercial launch in CE markets LOPAIN3 CONFIDENTIAL | APM008 rev. A; DCO0029 PATIENT ENROLLMENT PLAN CURRENT SITE LOCATIONS 27 ESTIMATED KEY MILESTONES DATE 1st patient enrolled Q1 2023 Gather data and submit Q2 2025 CE Mark approval Q3 2025 Product Launch Q4 2025 0 0 1 1 3 3 3 3 3 3 3 0 1 1 2 2 3 3 4 3Q 22 4Q 22 1Q 23 2Q 23 3Q 23 4Q 23 1Q 24 2Q 24 3Q 24 4Q 24 1Q 25 LOPAIN3 Georgia Paraguay (1) LOPAIN3 is considered a “First in Human Study” due to new indications (herniation patient population) that are different than th e NUCLEUS 181 and LOPAIN1/2 study.
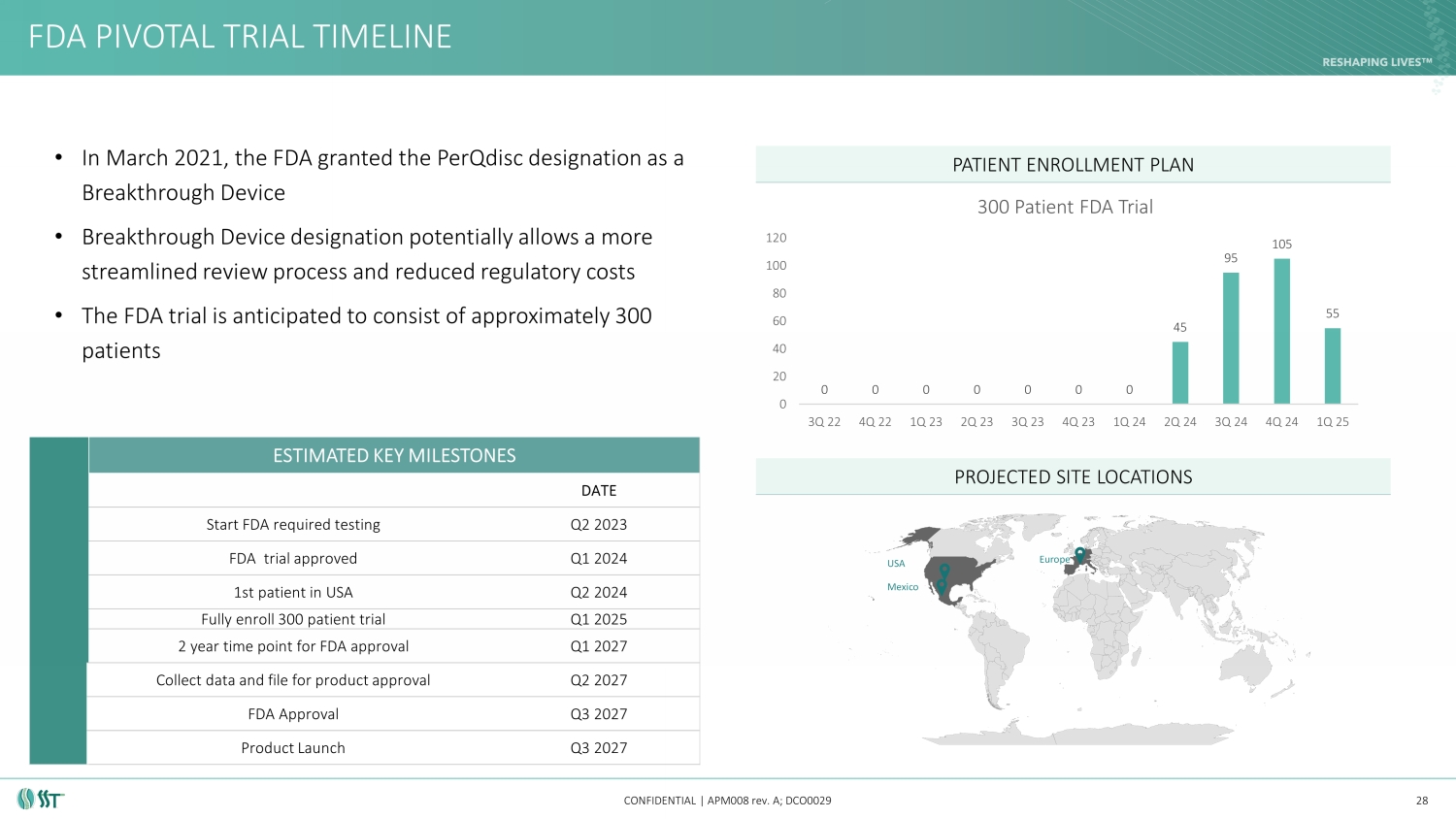
• In March 2021, the FDA granted the PerQdisc designation as a Breakthrough Device • Breakthrough Device designation potentially allows a more streamlined review process and reduced regulatory costs • The FDA trial will consist of 300 patients FDA PIVOTAL TRIAL TIMELINE CONFIDENTIAL | APM008 rev. A; DCO0029 PATIENT ENROLLMENT PLAN 28 ESTIMATED KEY MILESTONES DATE Start FDA required testing Q2 2023 FDA trial approved Q1 2024 1st patient in USA Q2 2024 Fully enroll 300 patient trial Q1 2025 2 year time point for FDA approval Q1 2027 Collect data and file for product approval Q2 2027 FDA Approval Q3 2027 Product Launch Q3 2027 PROJECTED SITE LOCATIONS 0 0 0 0 0 0 0 45 95 105 55 0 20 40 60 80 100 120 3Q 22 4Q 22 1Q 23 2Q 23 3Q 23 4Q 23 1Q 24 2Q 24 3Q 24 4Q 24 1Q 25 300 Patient FDA Trial Mexico USA Europe

Transaction Details
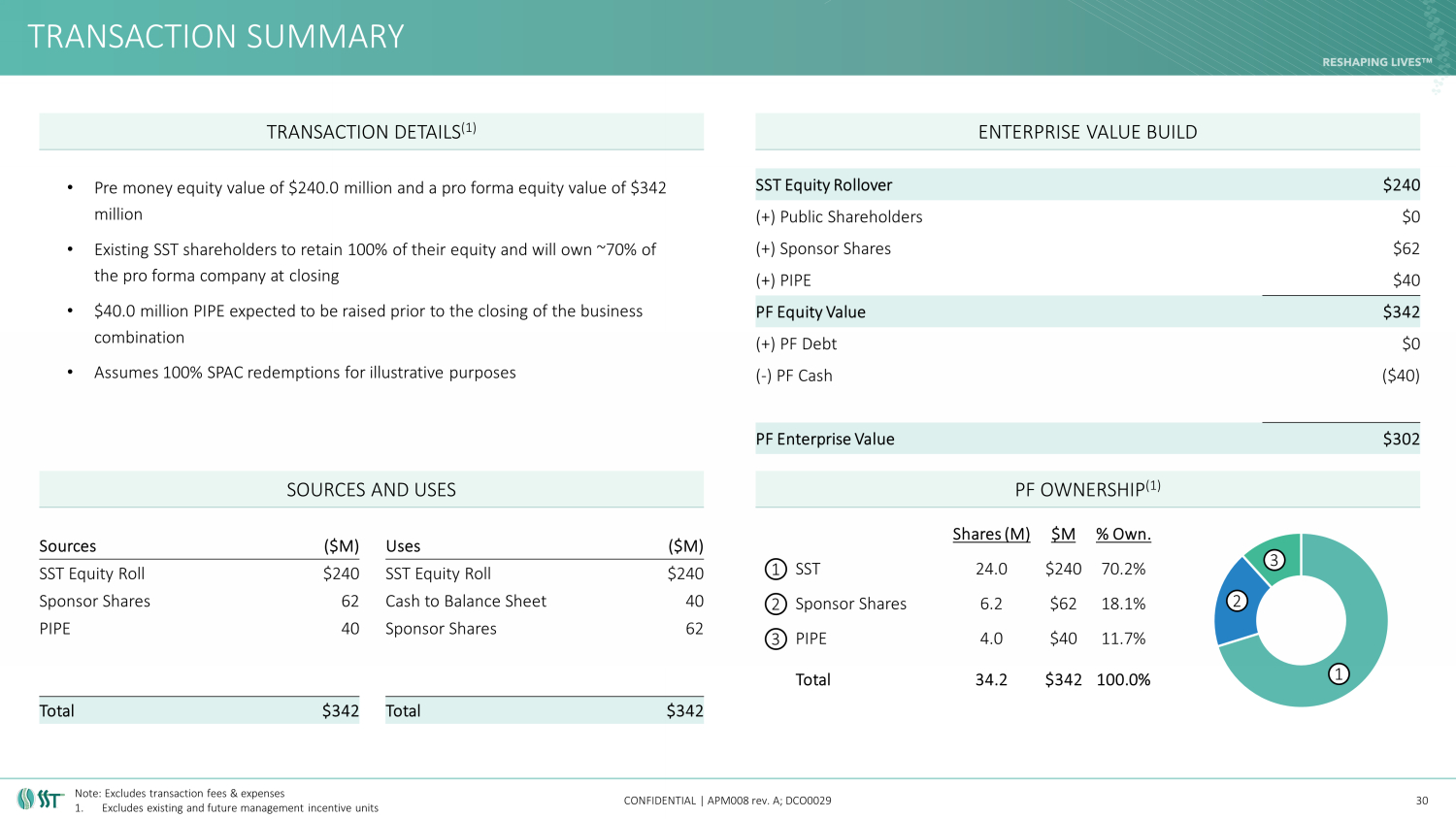
TRANSACTION SUMMARY CONFIDENTIAL | APM008 rev. A; DCO0029 • Pre money equity value of $240.0 million and a pro forma equity value of $342 million • Existing SST shareholders to retain 100% of their equity and will own ~70% of the pro forma company at closing • $40.0 million PIPE expected to be raised prior to the closing of the business combination • Assumes 100% SPAC redemptions for illustrative purposes 30 TRANSACTION DETAILS (1) ENTERPRISE VALUE BUILD SOURCES AND USES PF OWNERSHIP (1) SST Equity Rollover $240 (+) Public Shareholders $0 (+) Sponsor Shares $62 (+) PIPE $40 PF Equity Value $342 (+) PF Debt $0 ( - ) PF Cash ($40) PF Enterprise Value $302 Sources ($M) Uses ($M) SST Equity Roll $240 SST Equity Roll $240 Sponsor Shares 62 Cash to Balance Sheet 40 PIPE 40 Sponsor Shares 62 Total $342 Total $342 Shares (M) $M % Own. SST 24.0 $240 70.2% Sponsor Shares 6.2 $62 18.1% PIPE 4.0 $40 11.7% Total 34.2 $342 100.0% 1 2 3 1 2 3 Note: Excludes transaction fees & expenses 1. Excludes existing and future management incentive units

Appendix Additional Company Background

IRELAND MANUFACTURING CONFIDENTIAL | APM008 rev. A; DCO0029 32 VERTICALLY INTEGRATED • SST designs, develops and manufactures its orthopedic implants in its 9,395 sq ft facility in Kilkenny, Ireland • The facility is ISO 13485 Certified ISO 13485 Certified
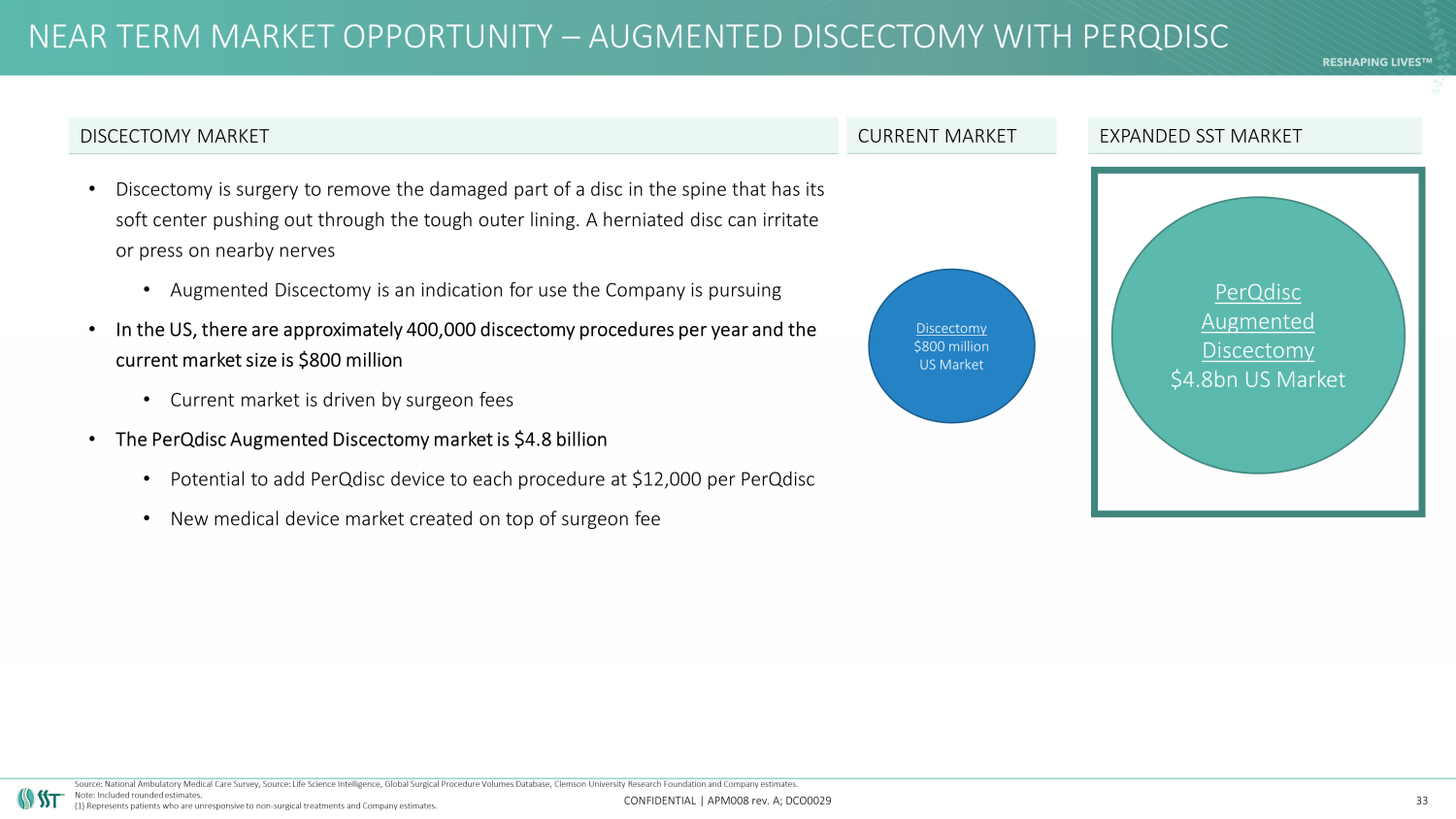
NEAR TERM MARKET OPPORTUNITY – AUGMENTED DISCECTOMY WITH PERQDISC CONFIDENTIAL | APM008 rev. A; DCO0029 • Discectomy is surgery to remove the damaged part of a disc in the spine that has its soft center pushing out through the tough outer lining. A herniated disc can irritate or press on nearby nerves • Augmented Discectomy is an indication for use the Company is pursuing • In the US, there are approximately 400,000 discectomy procedures per year and the current market size is $800 million • Current market is driven by surgeon fees • The PerQdisc Augmented Discectomy market is $4.8 billion • Potential to add PerQdisc device to each procedure at $12,000 per PerQdisc • New medical device market created on top of surgeon fee 33 DISCECTOMY MARKET Discectomy $800 million US Market EXPANDED SST MARKET PerQdisc Augmented Discectomy $4.8bn US Market CURRENT MARKET Source: National Ambulatory Medical Care Survey, Source: Life Science Intelligence, Global Surgical Procedure Volumes Databas e, Clemson University Research Foundation and Company estimates. Note: Included rounded estimates. (1) Represents patients who are unresponsive to non - surgical treatments and Company estimates.
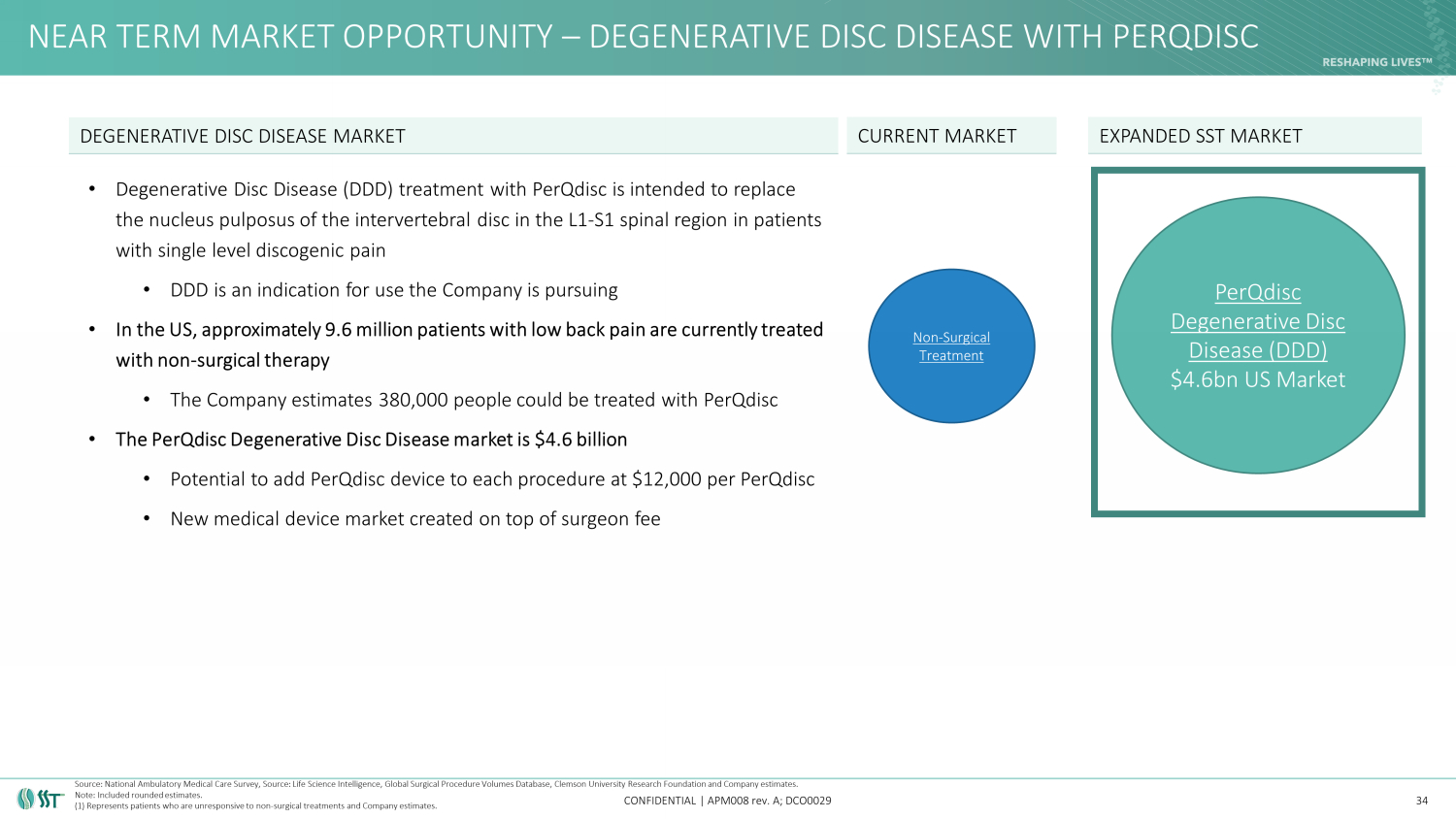
NEAR TERM MARKET OPPORTUNITY – DEGENERATIVE DISC DISEASE WITH PERQDISC CONFIDENTIAL | APM008 rev. A; DCO0029 • Degenerative Disc Disease (DDD) treatment with PerQdisc is intended to replace the nucleus pulposus of the intervertebral disc in the L1 - S1 spinal region in patients with single level discogenic pain • DDD is an indication for use the Company is pursuing • In the US, approximately 9.6 million patients with low back pain are currently treated with non - surgical therapy • The Company estimates 380,000 people could be treated with PerQdisc • The PerQdisc Degenerative Disc Disease market is $4.6 billion • Potential to add PerQdisc device to each procedure at $12,000 per PerQdisc • New medical device market created on top of surgeon fee 34 DEGENERATIVE DISC DISEASE MARKET Non - Surgical Treatment EXPANDED SST MARKET PerQdisc Degenerative Disc Disease (DDD) $4.6bn US Market CURRENT MARKET Source: National Ambulatory Medical Care Survey, Source: Life Science Intelligence, Global Surgical Procedure Volumes Databas e, Clemson University Research Foundation and Company estimates. Note: Included rounded estimates. (1) Represents patients who are unresponsive to non - surgical treatments and Company estimates.
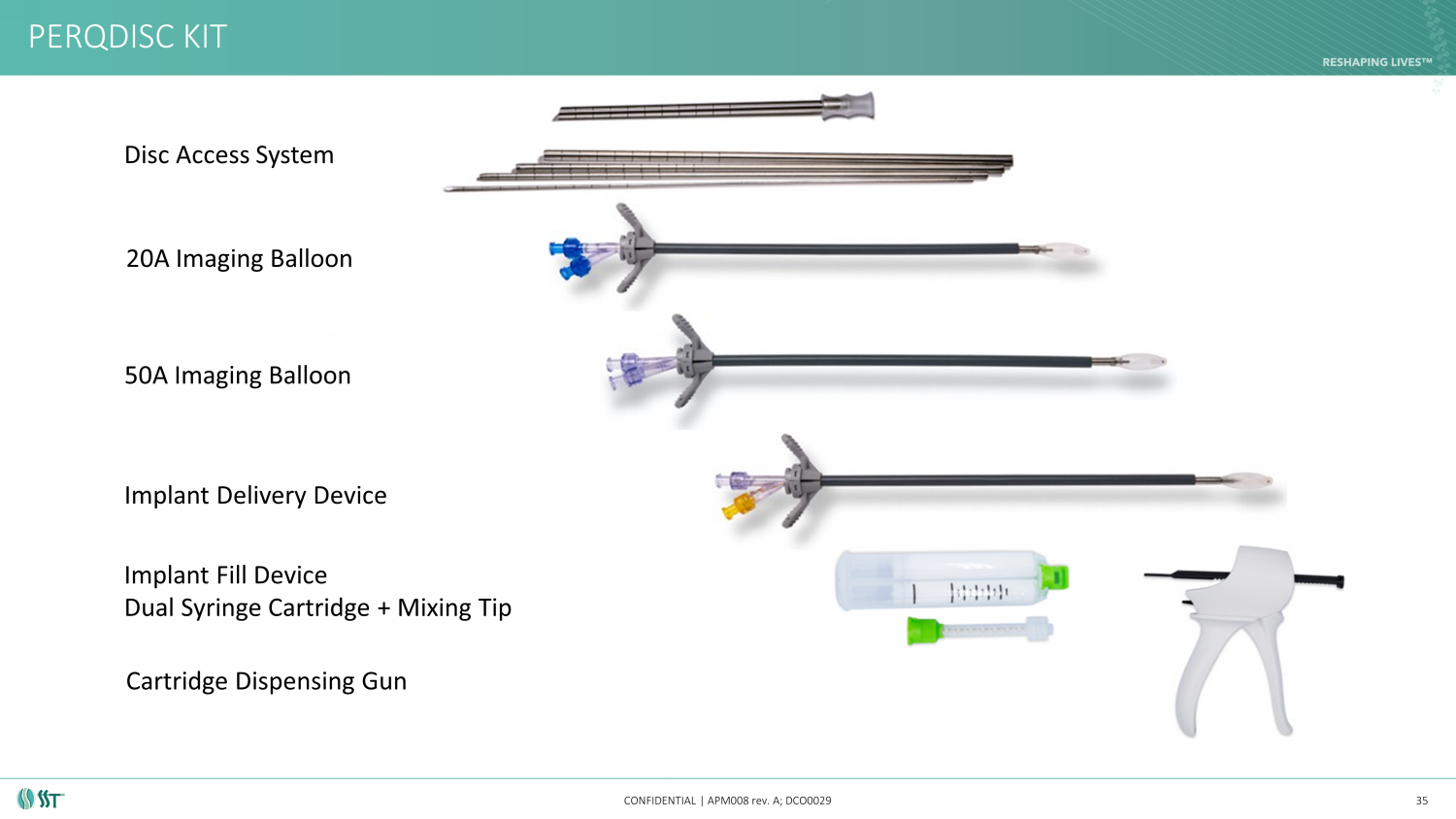
PERQDISC KIT CONFIDENTIAL | APM008 rev. A; DCO0029 35 Disc Access System 20A Imaging Balloon 50A Imaging Balloon Implant Delivery Device Implant Fill Device Dual Syringe Cartridge + Mixing Tip Cartridge Dispensing Gun
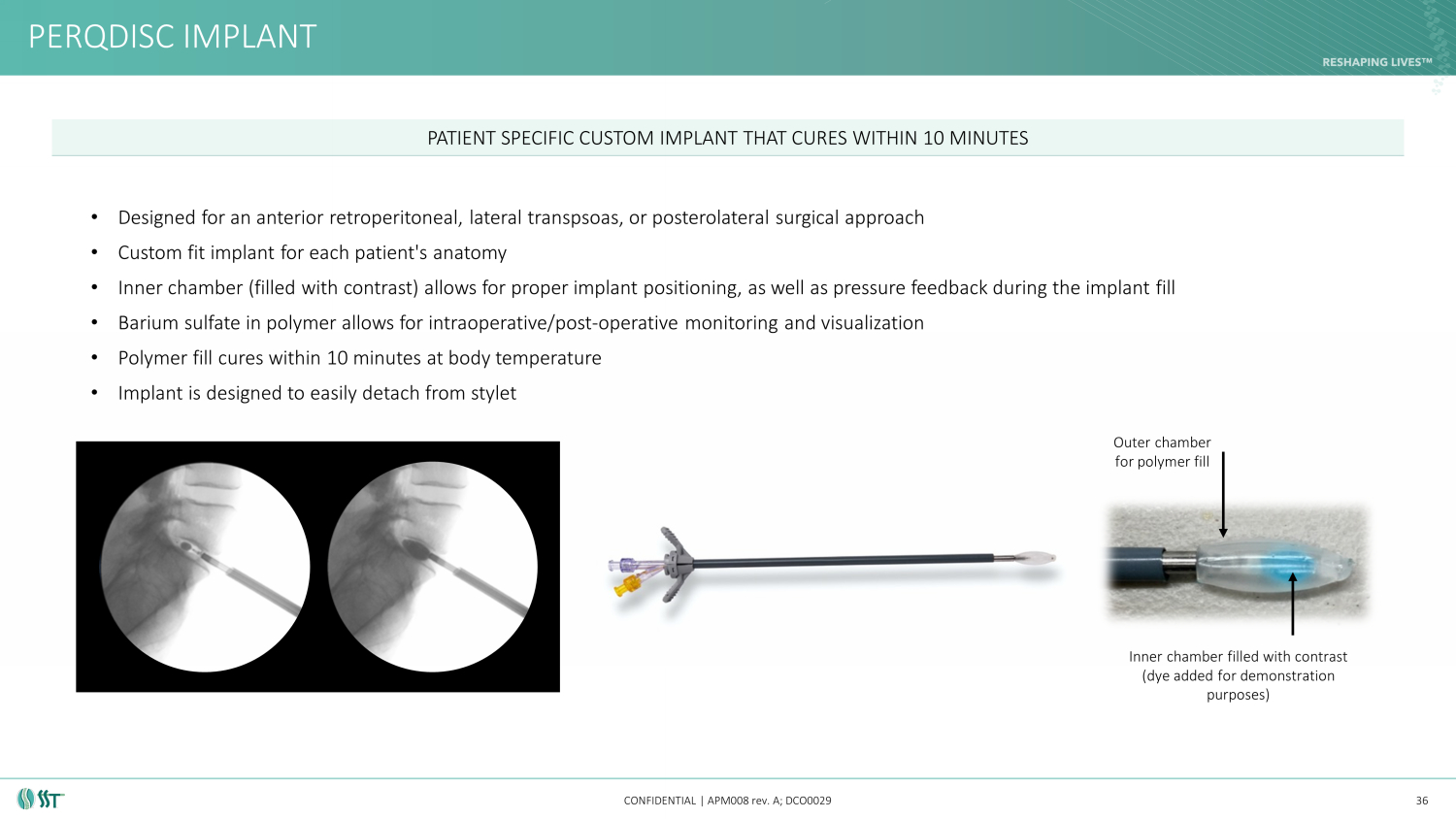
PERQDISC IMPLANT CONFIDENTIAL | APM008 rev. A; DCO0029 Outer chamber for polymer fill Inner chamber filled with contrast (dye added for demonstration purposes) 36 • Designed for an anterior retroperitoneal, lateral transpsoas , or posterolateral surgical approach • Custom fit implant for each patient's anatomy • Inner chamber (filled with contrast) allows for proper implant positioning, as well as pressure feedback during the implant f ill • Barium sulfate in polymer allows for intraoperative/post - operative monitoring and visualization • Polymer fill cures within 10 minutes at body temperature • Implant is designed to easily detach from stylet PATIENT SPECIFIC CUSTOM IMPLANT THAT CURES WITHIN 10 MINUTES
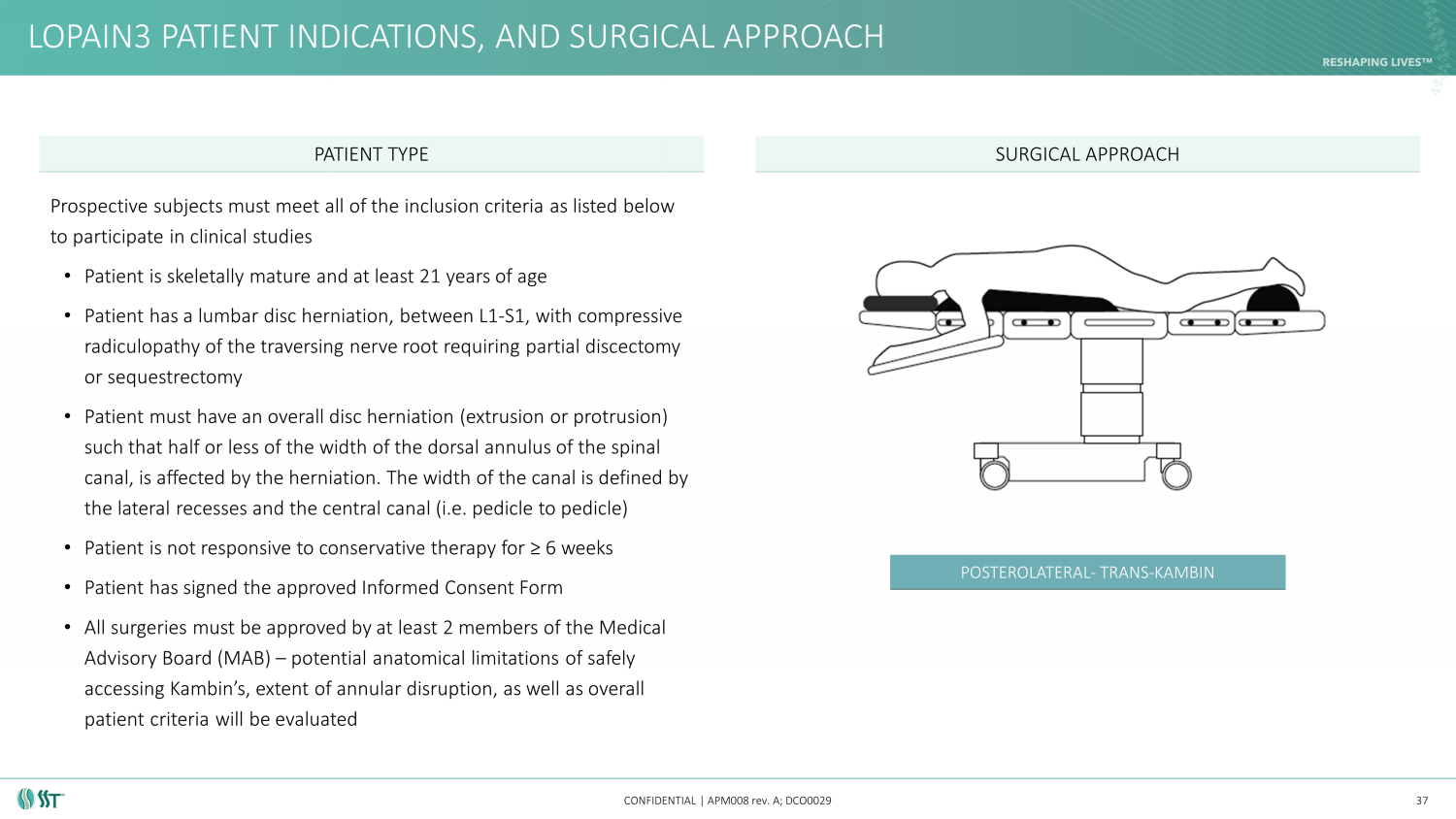
LOPAIN3 PATIENT INDICATIONS, AND SURGICAL APPROACH CONFIDENTIAL | APM008 rev. A; DCO0029 37 PATIENT TYPE SURGICAL APPROACH POSTEROLATERAL - TRANS - KAMBIN Prospective subjects must meet all of the inclusion criteria as listed below to participate in clinical studies • Patient is skeletally mature and at least 21 years of age • Patient has a lumbar disc herniation, between L1 - S1, with compressive radiculopathy of the traversing nerve root requiring partial discectomy or sequestrectomy • Patient must have an overall disc herniation (extrusion or protrusion) such that half or less of the width of the dorsal annulus of the spinal canal, is affected by the herniation. The width of the canal is defined by the lateral recesses and the central canal (i.e. pedicle to pedicle) • Patient is not responsive to conservative therapy for ≥ 6 weeks • Patient has signed the approved Informed Consent Form • All surgeries must be approved by at least 2 members of the Medical Advisory Board (MAB) – potential anatomical limitations of safely accessing Kambin’s , extent of annular disruption, as well as overall patient criteria will be evaluated
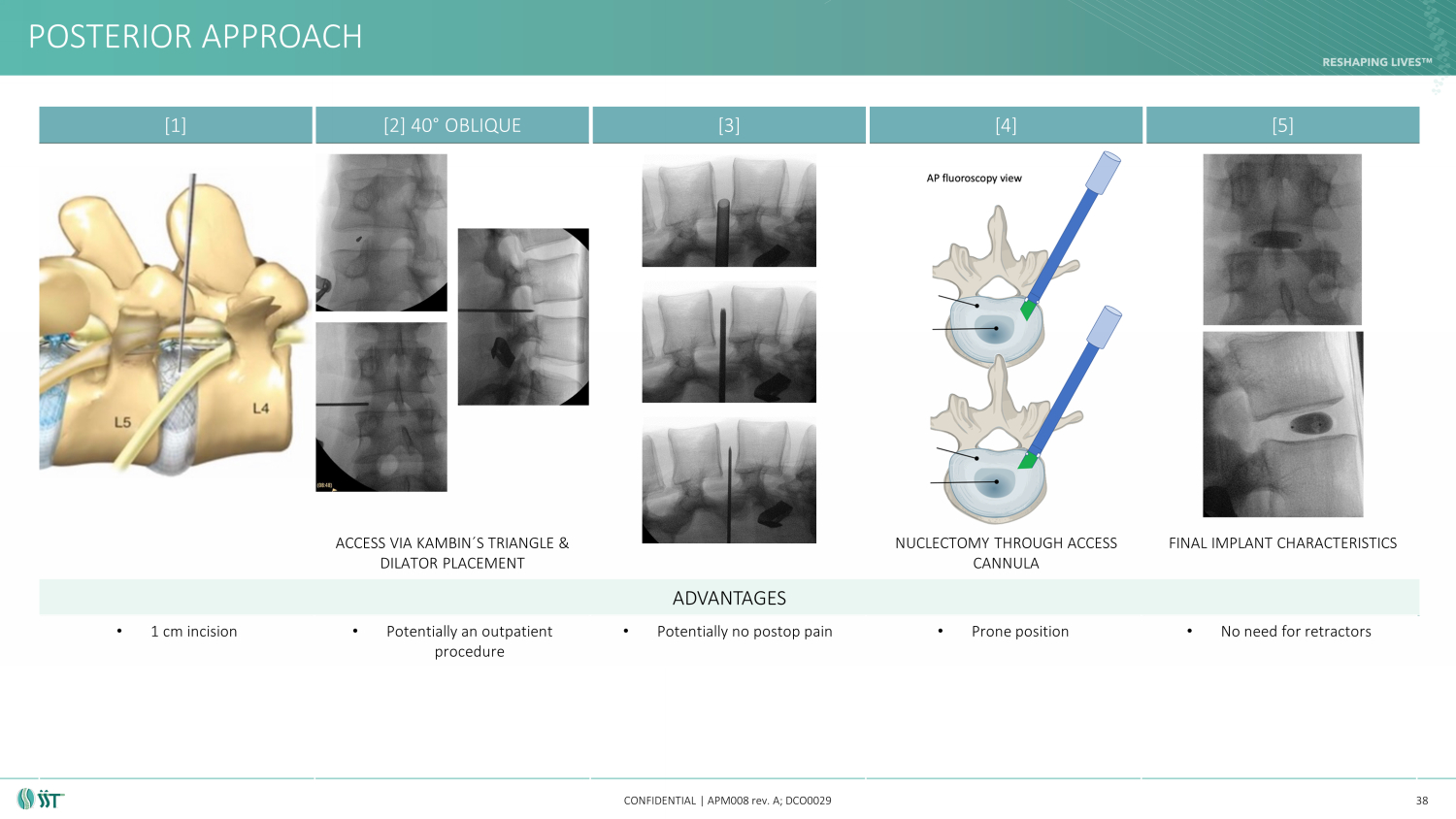
POSTERIOR APPROACH CONFIDENTIAL | APM008 rev. A; DCO0029 38 ADVANTAGES • 1 cm incision • Potentially an outpatient procedure • Potentially no postop pain • Prone position • No need for retractors [1] [2] 40 ° OBLIQUE [3] [4] [5] ACCESS VIA KAMBIN ´ S TRIANGLE & DILATOR PLACEMENT NUCLECTOMY THROUGH ACCESS CANNULA FINAL IMPLANT CHARACTERISTICS
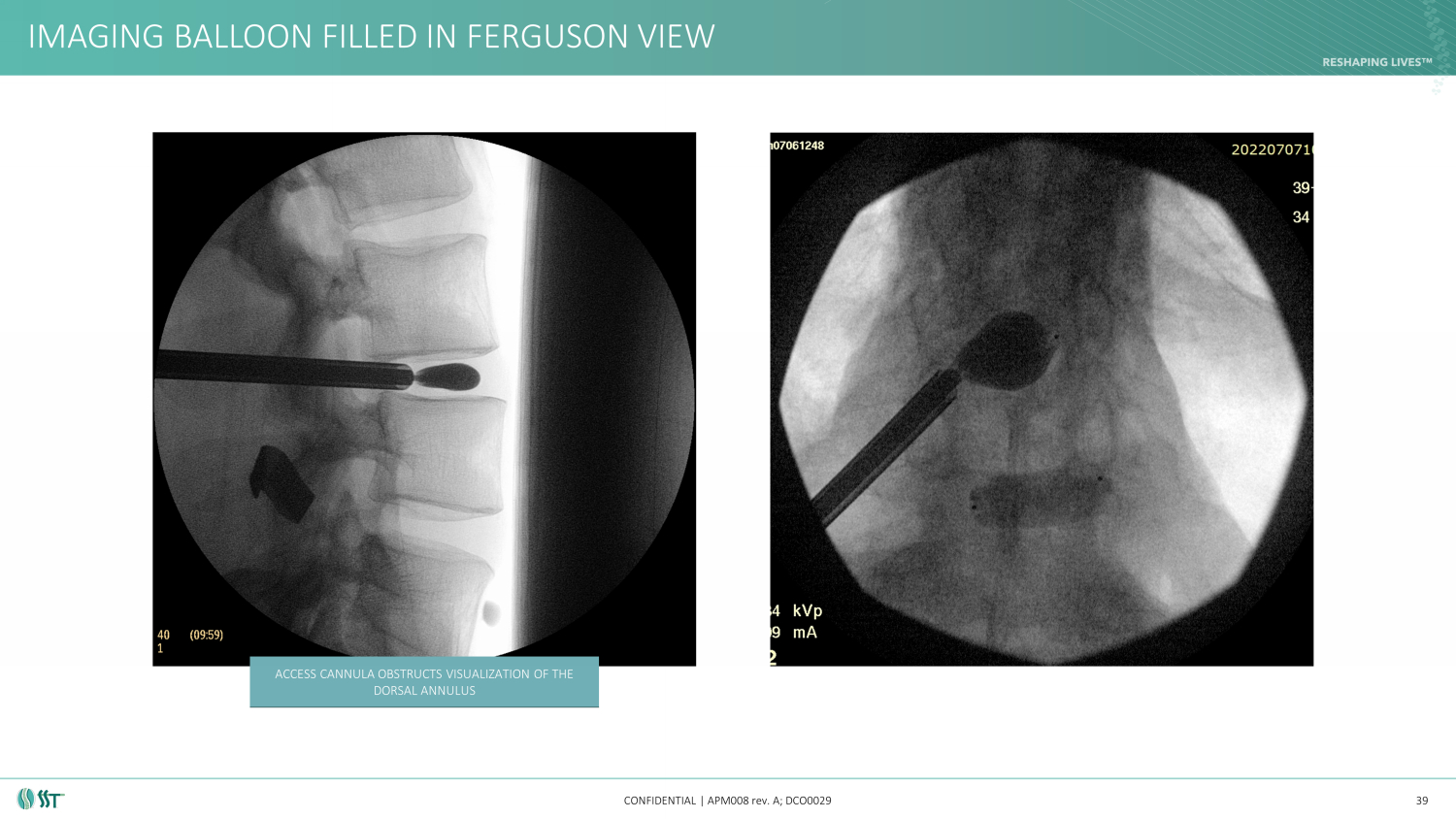
IMAGING BALLOON FILLED IN FERGUSON VIEW CONFIDENTIAL | APM008 rev. A; DCO0029 39 ACCESS CANNULA OBSTRUCTS VISUALIZATION OF THE DORSAL ANNULUS
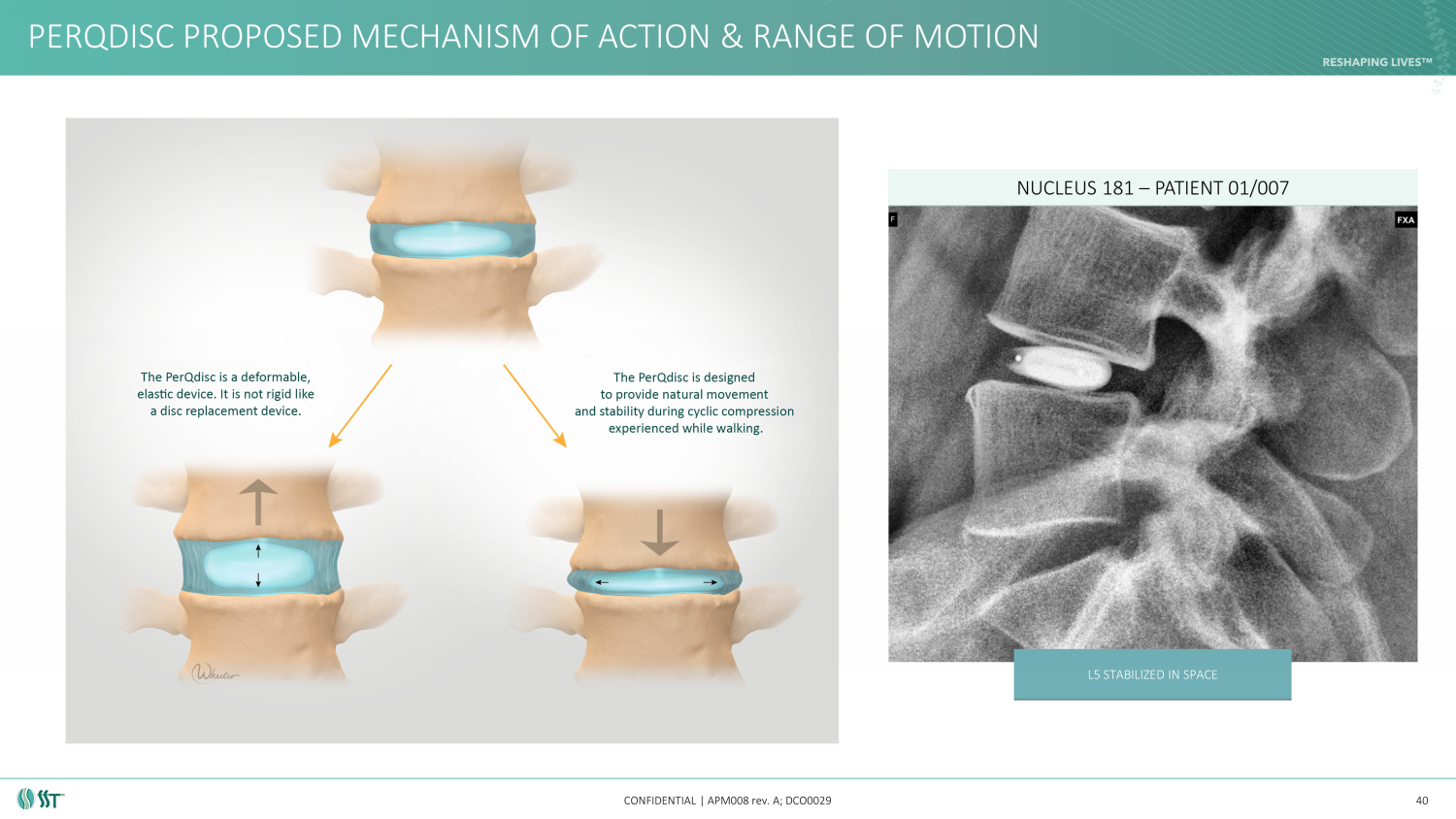
PERQDISC PROPOSED MECHANISM OF ACTION & RANGE OF MOTION CONFIDENTIAL | APM008 rev. A; DCO0029 40 NUCLEUS 181 – PATIENT 01/007 L5 STABILIZED IN SPACE

Appendix Sponsor Background and Case Studies

• Founder & CEO of Running M Capital and Co - Managing Partner of Mays Family Enterprises • Various leadership roles including Vice Chairman, President and CFO of Clear Channel Communications • Guided strategic direction of Clear Channel Entertainment and oversaw spinout into Live Nation. Served as Chairman of Live Nation from its inception until its merger with Ticket Master • Board of Live Nation Entertainment, Digital Defense, BuildGroup Technology Fund, Live Undiscovered Music (LÜM), Mind Science Foundation and the Mays Family Foundation • Previously served on the boards of CNET, XM Satellite Radio, and American Tower • MBA from Harvard Business School and BBA from University of Texas • Founder of Bluewater Ventures, a family office focused on investing in the technology, media, telecom and entertainment industries • Completed over 50 acquisitions, divestitures and corporate restructurings and arranged over $18 billion in strategic equity investments. He has successfully led operational restructurings in companies varying in size from 500 to 25,000 • Served on the board of over 25 companies. Previously CFO for NTL Inc (now Virgin Media), prior leadership roles at Cellular Communications, Iesy GMBH and Cablecom GMBH • Board of Quantum Earth and Saros • MBA from Harvard Business School and BA from Georgetown RELEVANT EXPERIENCE RELEVANT EXPERIENCE LEADING SPONSOR TEAM: INVESTORS AND OPERATORS CONFIDENTIAL | APM008 rev. A; DCO0029 42 RANDALL MAYS CO - CHAIRMAN, CO - CHIEF EXECUTIVE OFFICER AND CHIEF FINANCIAL OFF ICER JOHN GREGG CO - CHAIRMAN AND CO - CHIEF EXECUTIVE OFFICER
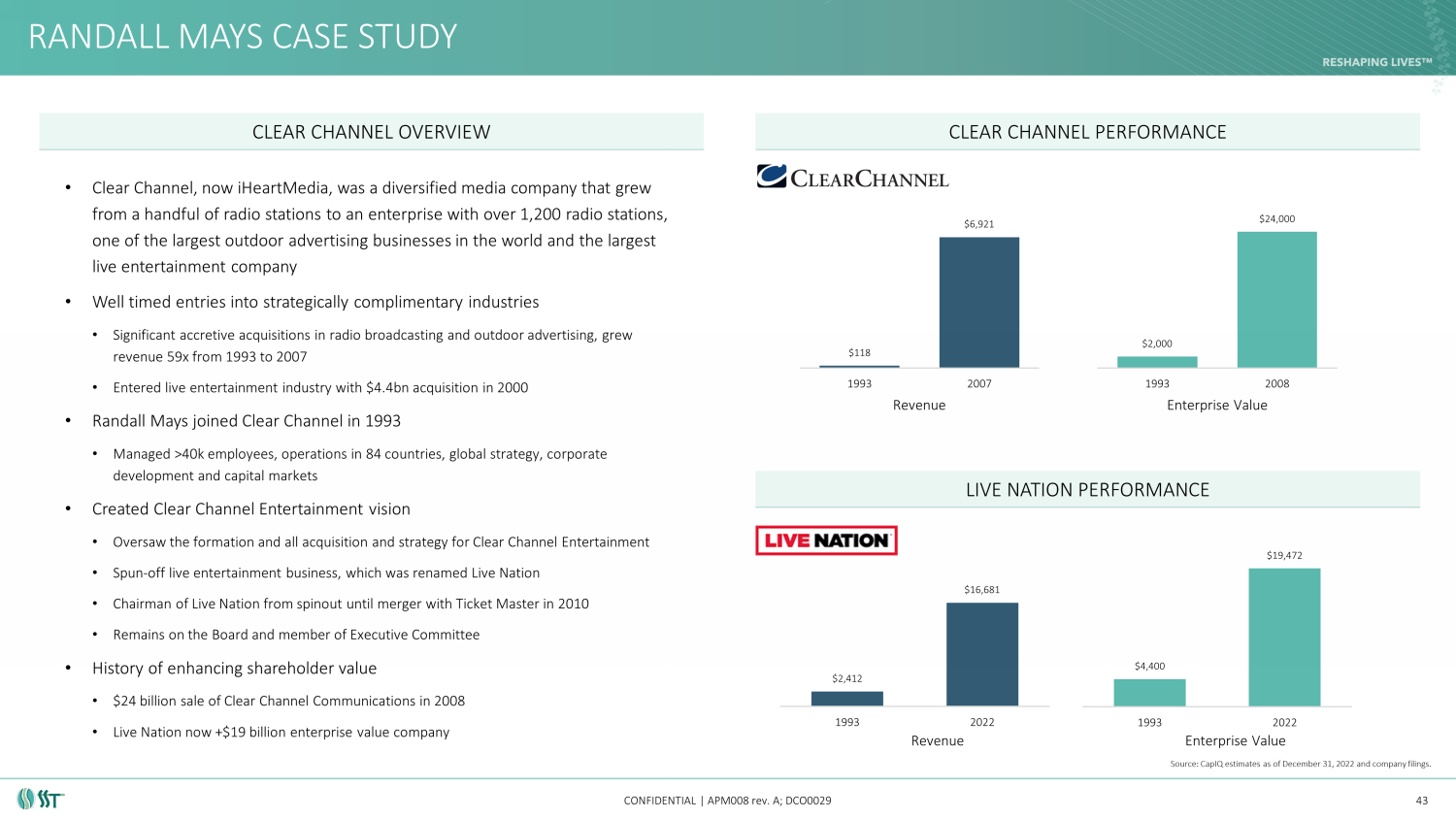
RANDALL MAYS CASE STUDY CONFIDENTIAL | APM008 rev. A; DCO0029 $2,412 $16,681 1993 2022 $118 $6,921 1993 2007 $2,000 $24,000 1993 2008 Revenue Enterprise Value $4,400 $19,472 1993 2022 Source: CapIQ estimates as of December 31, 2022 and company filings. 43 • Clear Channel, now iHeartMedia, was a diversified media company that grew from a handful of radio stations to an enterprise with over 1,200 radio stations, one of the largest outdoor advertising businesses in the world and the largest live entertainment company • Well timed entries into strategically complimentary industries • Significant accretive acquisitions in radio broadcasting and outdoor advertising, grew revenue 59x from 1993 to 2007 • Entered live entertainment industry with $4.4bn acquisition in 2000 • Randall Mays joined Clear Channel in 1993 • Managed >40k employees, operations in 84 countries, global strategy, corporate development and capital markets • Created Clear Channel Entertainment vision • Oversaw the formation and all acquisition and strategy for Clear Channel Entertainment • Spun - off live entertainment business, which was renamed Live Nation • Chairman of Live Nation from spinout until merger with Ticket Master in 2010 • Remains on the Board and member of Executive Committee • History of enhancing shareholder value • $24 billion sale of Clear Channel Communications in 2008 • Live Nation now +$19 billion enterprise value company CLEAR CHANNEL OVERVIEW CLEAR CHANNEL PERFORMANCE LIVE NATION PERFORMANCE Revenue Enterprise Value
JOHN GREGG CASE STUDY CONFIDENTIAL | APM008 rev. A; DCO0029 BUY AND BUILDS • Rolled up European broadcast transmissions / tower businesses as CFO • Tower site leasing, broadcast transmission, satellite, media, public safety communications and other network services in the U.K. and Ireland • Operational transformation and technology investments • Sold for GBP £1.3 billion / USD $2.5 billion to Macquarie CARVEOUTS AND OPERATIONAL TURNAROUNDS • Defense division carveout from BAE Systems • Led operational turnaround as Chairman • Facility consolidations, eliminated significant excess liabilities, employee rationalizations • Successful sale to strategic investor • Led turnaround of Cablecom, Switzerland’s leading cable network provider • Partnered with Apollo, Soros Private Equity Partners • Successful sale to Liberty Global for CHF 2.8 billion / USD $2.2 billion RECENT SIGNIFICANT INVESTMENTS • Artificial intelligence • Co - investments with sovereign wealth funds and Fortune 500 strategics 44

EXISTING BLUERIVER BOARD AND TEAM CONFIDENTIAL | APM008 rev. A; DCO0029 45 • Currently serves as Non - Executive Chairman of the Board of Pershing Square Holdings, Ltd • Experienced private equity investment professional. Former director of Providence Equity Partners in London and was one of the partners responsible for investing a $2.8 billion fund in telecom and media companies in Europe • Prior roles at Electra Partners, Morgan Stanley and Bain and Company • MBA from Harvard Business School • Served as a U.S. Senator from New Hampshire from 2003 to 2009 • Member of the Committees on Banking, Commerce, Finance and Foreign Relations, and he was appointed the Congressional Representative to the United Nations General Assembly • Former director of Time Warner Cable, Director of Boston Scientific since 2009, Council member of Lloyd’s of London since 2019 • MBA from Harvard Business School • Formerly the President and CFO of SoftBank Group International • Led deals for SoftBank including Sprint and T - Mobile merger, acquisition of ARM Holdings Plc, sale of Supercell Oy to Tencent, restructuring of SoftBank’s Yahoo Japan stake • Former board member of ARM Holdings, Fortress Investment Group, Brightstar Corp, SoftBank Energy, SoFi , SoftBank Group Capital and Airtel Africa • Over 30 years of investment banking, capital markets and investment experience in New York, London and Hong Kong • Experienced private equity investment professional. Private equity roles at Westhook Capital, aPriori Capital Partners and DLJ Merchant Banking Partners where he was responsible for sourcing, executing and managing investments • Prior roles at Credit Suisse and Barclays Capital • BBA from the Ross School of Business at the University of Michigan RELEVANT EXPERIENCE RELEVANT EXPERIENCE RELEVANT EXPERIENCE RELEVANT EXPERIENCE ANNE FARLOW SPAC DIRECTOR JOHN E. SUNUNU SPAC DIRECTOR ALOK SAMA SPAC DIRECTOR ERIC MEDINA MANAGING DIRECTOR, HEAD OF FINANCE AND CORPORATE DEVELOPMENT
PRELIMINARY RISK FACTORS The list below of risk factors has been prepared as part of the Business Combination. The risks presented below are certain o f t he general risks related to the business of SST, BlueRiver and the combined company, and such list is not exhaustive. The lis t b elow is qualified in its entirety by disclosures contained in future documents filed or furnished by BlueRiver, SST and the combined company with the SEC . If SST, BlueRiver and the combined company cannot address any of the following risks and uncertainties effectively, or any oth er risks and difficulties that may arise in the future, their business, financial condition, or results of operations could be materially and adversely af fected. The risks described below are not the only ones SST, BlueRiver and the combined company face. Additional risks that S ST, BlueRiver and the combined company currently do not know about or that they currently believe to be immaterial may also impair their business, financial co ndition or results of operations. You should review this Presentation and perform your own due diligence prior to making an i nve stment in BlueRiver, SST and the combined company. Risks Related to SST’s Business • The PerQdisc Nucleus Replacement System indicated for degenerative disc disease was granted a CE Mark in 2021 but the Mark was suspended b y SST’s Notified Body. SST is working with the Notified Body to reinstate the CE Mark. SST is required to collect clinical data on significantly more patients in order to obtain a statistically - significant data set and receive CE Mark approval under the Regul ation (EU) 2017/745 (MDR), and both the number of additional required patients and the length of time necessary to complete t his process is uncertain. CE Mark approval under the MDR regulations is highly uncertain. • SST may be required to successfully complete an FDA clinical trial before potential strategic partners will consider a transa cti on with the Company, and SST cannot provide any assurance that the FDA will authorize a clinical trial of SST’s products or t hat SST will be able to complete such a trial. The FDA’s approval of SST’s clinical trial is highly uncertain. • SST may never successfully commercialize its lumbar implant product. Success in the long - term medical device implant sector is h ighly dependent on market adoption by surgeons and surgical facilities. Even if SST is successful in obtaining CE Mark and FD A a pproval of its products, SST cannot offer any assurance that its products will be adopted by surgeons or added by surgical facilities. Moreo ver , SST’s commercialization efforts will be dependent on surgeons and facilities being reimbursed at favorable rates for the us e o f SST’s products. No assurance can be given that reimbursement approvals will be granted by government or private insurance programs for SST’s pro duc ts and procedures. • SST is very thinly capitalized, and its continued operations are dependent upon receiving the funds raised in this offering. • SST is highly dependent on a core group of employees. The competition for such employees is high and SST cannot offer assuran ce that it will be able to retain such employees or recruit additional employees that will be needed for the commercialization o f S ST’s products. Risks Related to SST’s Common Stock Following the Business Combination • The market price of SST’s common stock could be volatile, and you could lose all or part of your investment. • SST expects its quarterly revenues and operating results to fluctuate. If SST fails in future periods to meet its publicly an nou nced financial guidance or the expectations of securities analysts or investors, the market price of SST’s common stock could de cline substantially. • SST does not intend to pay dividends on its common stock. • If securities or industry analysts do not publish research or publish inaccurate or unfavorable research about SST’s business , i ts stock price and trading volume could decline. • The requirements of being a public company, including compliance with the reporting requirements of the Securities Exchange A ct of 1934, as amended and The New York Stock Exchange, will require significant resources, increase SST’s costs and distract it s m anagement, and SST may be unable to comply with these requirements in a timely or cost - effective manner. • Any future sales or offerings of SST’s common stock may cause substantial dilution to SST’s stockholders and could cause the pri ce of its common stock to decline. Risks Related to the Business Combination • The consummation of the Business Combination is subject to a number of conditions and if those conditions are not satisfied o r w aived, the Business Combination Agreement may be terminated in accordance with its terms and the Business Combination may not be completed. • There is no guarantee that a BlueRiver shareholder’s decision as to whether to redeem its BlueRiver Class A ordinary shares f or a pro rata portion of the Trust Account will put the shareholder in a better or worse economic position. • If the Business Combination benefits do not meet the expectations of investors or securities analysts, the market price of BlueRiver’s securities or, following the consummation of the Business Combination, the combined company’s securities may decline. • Legal proceedings in connection with the Business Combination, the outcomes of which are uncertain, could delay or prevent th e c ompletion of the Business Combination. CONFIDENTIAL | APM008 rev. A; DCO0029 46

Thank You